Abstract
Objective: To examine the effects of short-term valproate treatment on human brain serotonin and dopamine function by means of challenge tests with ipsapirone, a partial agonist at 5-HT1A receptors, and apomorphine, a dopamine receptor agonist.
Design: Experimental challenge–rechallenge, within-subjects repeated measures, before and at the end of 14 days of treatment with valproate at a dosage of 625 mg/d (reached gradually over the first 5 days).
Participants: Eight healthy male volunteers (mean age 38 years) selected for good physical and mental health who were nonsmokers.
Outcome measures: Pharmacological probes were used to evaluate the effects of valproate. In the ipsapirone challenge, changes in adrenocorticotropic hormone (ACTH), cortisol and body temperature were measured, and in the apomorphine challenge, growth hormone (GH) and prolactin were the dependent variables.
Results: Valproate treatment did not significantly alter the ACTH, cortisol or body temperature responses to ipsapirone (20 mg by mouth), which reached equivalent plasma levels at each challenge. Similarly, valproate treatment did not alter the GH or prolactin responses to apomorphine (5 μg/kg subcutaneously).
Conclusions: These results suggest that short-term treatment with valproate at a dose of 625 mg/d does not alter hypothalamic or pituitary 5-HT1A or dopamine receptor responses to challenges with ipsapirone and apomorphine, respectively.
Introduction
Valproic acid (2-propylpentanoic acid, 2-propylvaleric acid, di-n-propylacetic acid) is used in the treatment of a number of neuropsychiatric conditions, including bipolar disorder. Valproate (used here to refer to valproic acid, sodium valproate and divalproex sodium) has been shown to be effective in the treatment of acute mania1,2 and in the prophylaxis of bipolar disorder, particularly in those subtypes of bipolar disorder for which lithium is relatively ineffective.3,4 Evidence supporting the efficacy of valproate in the long-term prophylaxis of bipolar disorder is growing,5,6 although Bowden et al7 recently reported that valproate had no greater efficacy than placebo in preventing the recurrence of mania. The mechanisms by which valproate may exert mood-stabilizing effects are not yet elucidated. Studies to date indicate that valproate has a wide range of effects on neurotransmitters, hormones and subcellular processes in the mammalian central nervous system (CNS). Valproate’s proposed actions on discrete neurotransmitter systems include effects on gamma-aminobutyric acid (GABA),8,9 serotonin (5-HT),10,11 dopamine (DA),12–15 glutamate16 and aspartate;17,18 our study examines the effects of valproate on 5-HT and DA function in the human CNS.
Serotonin mediates many physiologic processes19 and appears to be involved in the pathogenesis of depression, mania, migraine and myoclonic epilepsy, among other conditions. Many of these disorders benefit from treatment with valproate.10,20–22 Altered DA function has also been implicated in several neurologic and psychiatric illnesses, and there is evidence that it is involved in the pathogenesis of mania.23,24 Several animal studies have been conducted to investigate the impact of valproate on serotonergic neurotransmission, the preponderance of which suggest that valproate increases 5-HT levels and turnover in at least some areas of the mammalian brain.13,25–29 Only a few studies have been conducted in humans, however.10,30 Similarly, although valproate has been shown to increase brain concentrations of DA and its major metabolites in animal models, 13–15,31 few studies have attempted to examine such effects in humans.12,32 There is clearly a need for more data on the impact of valproate treatment on serotonergic and dopaminergic neurotransmission in humans.
A minimally invasive way of examining the effects of valproate on discrete neurotransmitter systems in humans is provided by the neuroendocrine challenge.33 A number of pharmacologic probes are available for use in human subjects; these can safely be used to test the function of neurotransmitters, including 5-HT and DA. Central serotonergic function can be measured using either pre- or post-synaptic agonists, with varying specificity at different 5-HT receptor subtypes. Ipsapirone, a partial 5-HT1A receptor agonist, has high affinity and selectivity for 5-HT1A recognition sites and negligible affinity for the 5-HT1B, 5-HT1C, 5-HT1D, 5-HT2 and 5-HT3 subtypes.34 When given orally to humans, it causes a dose-dependent 5-HT1A receptor-mediated increase in adrenocorticotropic hormone (ACTH) and cortisol secretion, and a decrease in body temperature. 35 This response is blocked by 5-HT1A receptor antagonists (such as pindolol), suggesting that ipsapirone responses provide a valid index of 5-HT1A receptor sensitivity in humans.36 Central dopaminergic function can be assessed by DA receptor antagonists or agonists. Apomorphine is a DA receptor agonist,37,38 which when administered subcutaneously in humans, causes DA receptor-mediated increases in plasma growth hormone (GH) levels and decreases in prolactin levels.39 Although at high doses (i.e., 5–6 mg subcutaneously), apomorphine has emetic effects,40 it is less likely to have such effects at the much lower doses used in neuroendocrine assessment (i.e., 5 μg/kg subcutaneously). 41 When considering the use of ipsapirone and apomorphine as neuroendocrine probes to investigate the impact of valproate on human serotonergic and dopaminergic function, it is important to note that Abraham et al42 found that the administration of 600 mg/d of valproate to healthy human subjects for 3 weeks had no effects on basal or insulin-induced secretion of ACTH, cortisol, prolactin or GH. Thus, at this dose of valproate (600 mg/d), the responses to subsequent challenges can be assessed without the concern of the nonspecific effects of valproate on these outcome measures of interest.
We examined the effects of the administration of valproate for 2 weeks in healthy male volunteers by means of neuroendocrine challenge tests with ipsapirone and apomorphine.
Methods
Eight healthy male subjects provided written informed consent for this study, which was approved by the Research Ethics Committee of the Royal Ottawa Hospital, Ottawa, Ont. Female subjects were excluded to eliminate any confounding effects of the menstrual cycle, given the experimental design that required identical tests to be performed 2 weeks apart. The age range of the subjects was restricted to 21–55 years. Subjects were required to be in good health. Satisfactory physical health in each potential subject was established by a careful medical history and physical examination, complete blood count, liver function tests, serum amylase test and electrocardiogram. Subjects were also required to have no history of psychiatric illness and to be in a good state of psychological health, as ascertained by means of an interview employing the Mini-International Neuropsychiatric Interview, version 2.1,43 and by each subject’s completion of a Beck Depression Inventory44 before and at the end of valproate treatment. The subjects were free of all medication and were nonsmokers. No alcohol was permitted during the study; coffee and other caffeinated beverages were permitted in moderation.
Sodium divalproex was administered over a period of 14 days. The starting dose of 250 mg/d, by mouth, was increased to 500 mg/d over the first 3 days, and then to 625 mg/d on the fifth day. During the 2-week period of drug treatment, subjects were contacted twice a week to inquire about their state of health, and the blood count and biochemical assessment were repeated at the end of the first week of treatment to detect any possible toxicity. Plasma valproate levels were determined at the end of the second week.
Subjects underwent the neuroendocrine challenge tests immediately before and then again at the end of valproate treatment. On both occasions, the 2 pharmacologic challenges were given on 2 successive days, with the apomorphine challenge first. Subjects were tested while reclining and awake. For the apomorphine challenge, subjects arrived at the testing room at 8:30 am after an overnight fast. A heparinized venous cannula was inserted into the antecubital vein at 8:45 am, and after a 15-min rest period, 3 samples of blood were taken at 15-min intervals for baseline determination of hormone levels. Apomorphine (5 μg/kg subcutaneously) was then administered at 9:30 am (for the second test, this was 12.5 hours after the last dose of valproate), and samples of blood continued to be taken every 15 min for the next 90 min. On the next day, subjects fasted after 8 am and arrived at 11:30 am for the ipsapirone challenge test. After 30 min of rest, the venous cannula was inserted, and the baseline sample of plasma and baseline oral temperature were obtained. Ipsapirone (20 mg, by mouth) was then given at 12 noon (for the second test, this was 4 hours after the last dose of valproate), and blood samples were taken 30, 45, 60, 75, 90, 105, 120, 150 and 180 min later. Oral temperature was measured at 30-min intervals throughout the test period, using a standard digital thermometer (QTest Basal Thermometer, Becton Dickinson Canada, Inc., Mississauga, Ont.).
Blood samples were collected in chilled tubes and immediately placed on ice; they were centrifuged and the plasma was frozen for subsequent analysis. Serum ACTH, cortisol, GH and prolactin levels were measured by Z.M. by means of radioimmunoassay using commercial kits (ICN Biomedicals, Inc., Costa Mesa, Calif.). Plasma ACTH and GH levels were assessed using a double antibody procedure (double 125I RIA kits), whereas plasma cortisol (125I RIA kit) and prolactin (125I IRMA Kit) levels were determined by means of a solid-phase (ImmuChem) radioimmunoassay. All assays were done in duplicate, and all samples for a particular hormone were analyzed in a single assay to avoid interassay variability. Ipsapirone and its metabolite, 1-(2-pyrimidinyl)-piperazine (1PP), were measured by M.F. by means of high-performance liquid chromatography with coulometric end-point detection and solid-phase extraction. An internal standard was used to monitor extraction recovery and detector response variation. Both intra- and inter-assay coefficients of variation were less than 10%, and the limit of detection was 0.5 μg/L when 1 mL of plasma was extracted. The effects of valproate treatment on the outcome measures of the challenge tests were determined by 3 methods: (1) repeated measures of analysis of variance (ANOVA), (2) comparison of the area under the curve (AUC) of each variable over time before and after valproate treatment using paired t-tests and (3) comparison of the maximal change in each variable (delta max) before and after valproate treatment by means of paired t-tests.
Results
The mean age, weight and body mass index of the 8 healthy male subjects were 38 years (standard deviation [SD] 12 yr, range 22–55 yr), 83.3 kg (SD 9.2 kg, range 71.9–97.5 kg), and 24.6 (SD 1.9, range 21.9–26.9), respectively. The mean plasma valproate concentration was 203 μmol/L (SD 83 μmol/L) at 12 hours after the previous dose.
During the ipsapirone challenge tests, the room temperature ranged from 22°C–25°C, and there was no difference between the mean temperatures on the test days before and during valproate treatment (23.0°C v. 23.25°C; t = 0.48, not significant [NS]). Furthermore, there was no evidence of depression either before or during valproate treatment (Beck Depression Inventory scores: 0.50 v. 0.88; t = 1.00, NS).
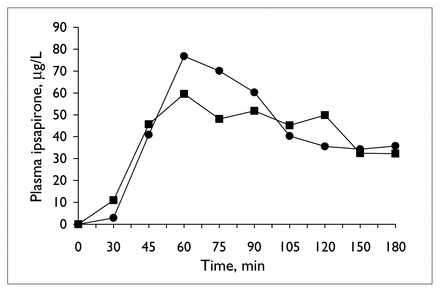
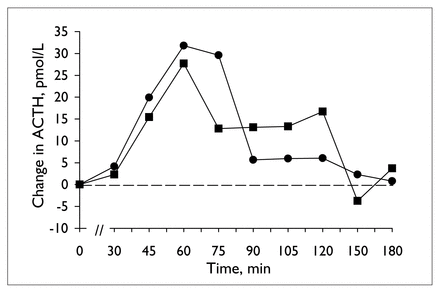
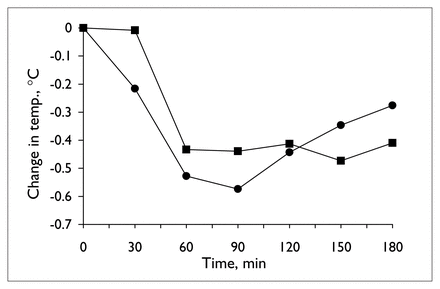
Table 1 provides details of the analysis of the neuroendocrine challenge tests before and during valproate treatment. Plasma ipsapirone levels did not differ between the 2 ipsapirone challenge tests (Table 1 and Fig. 1). Concentrations of the metabolite 1PP were generally below detectable limits and, hence, are not reported. The administration of ipsapirone produced reliable changes in plasma ACTH and cortisol and body temperature (Table 1). Treatment with 14 days of valproate, however, did not alter the responses to ipsapirone (Table 1, Fig. 2, Fig. 3). With the exception of a significant time by treatment interaction for temperature (Table 1, Fig. 3), there were no statistically significant differences between ACTH, cortisol or body temperature measured before and during treatment (Table 1). At first sight, the significant interaction of time and treatment for body temperature might suggest that there is an effect of valproate on ipsapirone-induced hypothermia. If the interaction were truly representative of an effect of valproate treatment, however, it would have been corroborated by the analyses of area under the curve and maximum temperature change. Since these were clearly not affected by valproate treatment, it can be concluded that the significant time by treatment interaction for body temperature is not indicative of an attenuated hypothermic response as a result of valproate treatment.
Mean ipsapirone plasma concentration during the ipsapirone challenge tests, before (circles) and at the end of 14 days of valproate treatment (squares).
Mean change in plasma adrenocorticotropic hormone (ACTH) concentration during ipsapirone challenge tests, before (circles) and at the end of 14 days of valproate treatment (squares).
Mean change in body temperature during ipsapirone challenge tests, before (circles) and at the end of 14 days of valproate treatment (squares).
Effects of valproate treatment on outcome of neuroendocrine challenge with ipsapirone and apomorphine
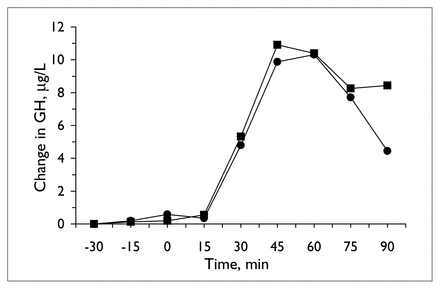
The apomorphine challenge also produced reliable changes in plasma hormones (Table 1, Fig. 4). Because GH may produce negative feedback on its own secretion, 33 one subject with a high baseline plasma level (above 3.75 μg/L) on one occasion was dropped from the analysis. Including this subject (in whom there was no evidence of inhibition of secretion of GH after the challenge) in the analysis, however, did not alter the findings; in both analyses, there was no change in the GH response with valproate treatment. Treatment with valproate also failed to affect the prolactin response (Table 1).
Mean change in plasma growth hormone (GH) concentration (for the 7 subjects with basal growth hormone levels below 3.75 μg/L) during apomorphine challenge tests, before (circles) and at the end of 14 days of valproate treatment (squares).
Thus, with the lone exception of the interaction between time and treatment for the hypothermic effect of ipsapirone, the neuroendocrine and hypothermic effects of challenge with ipsapirone and apomorphine were not significantly different before or during valproate treatment.
Discussion
Two weeks of treatment with valproate at doses up to 625 mg/d, by mouth, had no effect on ipsapirone-induced increases in ACTH and cortisol release. Furthermore, valproate treatment exerted no robust effect on ipsapirone-induced hypothermia. Although there was an interaction between time and treatment for temperature, the lack of any effect of valproate on maximal temperature change or on the area under the curve for temperature suggests that valproate treatment at a dose of up to 625 mg/d for 14 days does not attenuate the hypothermic response to ipsapirone. In addition, short-term treatment with valproate does not appear to have effects on the neuroendocrine response to apomorphine. Thus, there is little evidence from this study that the mood-stabilizing effects of valproate are mediated by its effects on 5-HT1A and DA receptors.
Valproate and the 5-HT1A receptor
Ipsapirone is a selective 5-HT1A receptor partial agonist that induces hypothermia and secretion of ACTH and cortisol.35 Ipsapirone produced these expected effects in our study, but valproate treatment failed to change the body temperature, ACTH and cortisol responses to ipsapirone.
Reports on the effects of valproate at the 5-HT1A receptor are limited and contradictory. Khaitan et al45 found that 21 days of valproate treatment had no effect on 8-OH-DPAT (a 5-HT1A receptor agonist) induced hypothermia in rats. However, Shiah et al30 found that valproate attenuated the hypothermic response to ipsapirone; in 10 healthy male volunteers, body temperature, ACTH and plasma cortisol levels and behavioural responses were measured during 2 ipsapirone challenge tests, 1 performed before and 1 at the end of 1 week of treatment with valproate (1000 mg/d). In this study, the hypothermia induced by ipsapirone appeared to be significantly attenuated by valproate treatment, whereas ACTH, plasma cortisol and behavioural responses were not affected.
The fact that our results differ from those of Shiah et al30 may be explained by several methodologic differences between the 2 studies. On average, our participants were 9 years older than those of the Shiah et al30 group and, thus, may have had less sensitive 5-HT1A receptors.46 Furthermore, the average weight of the participants differed between the 2 studies, resulting in significantly lower doses of valproate in our study in comparison with that of Shiah et al30 (7.5 mg/kg v. 13.9 mg/kg). This was reflected in the mean plasma levels achieved: 629 μmol/L (SD 131 μmol/L) in the study by Shiah et al30 compared with 203 μmol/L (SD 83 μmol/L) in our own study. At present, the relation between valproate dose, plasma valproate levels and therapeutic outcome in psychiatric disorders is unclear. 47,48 Bowden et al47 found that acutely manic patients treated with valproate at serum levels above 300 μmol/L were more likely to demonstrate improvement than those with lower plasma levels. Considerably lower plasma levels have, however, been shown to be effective in the treatment of cyclothymia and bipolar 2 disorder;49 a mean plasma level of 225 μmol/L was effective in most patients in the open-label trial. Nevertheless, the larger dose employed by Shiah et al30 may account for the differences in the hypothermic outcome measure if the effects of valproate on 5-HT1A receptors are dose-dependent. The duration of valproate treatment may also account for the differences between the 2 studies, for it is possible that there is an acute affect of valproate on the 5-HT1A receptors, which is measurable at 1 week, but which disappears by 14 days of treatment. It is also possible that desensitization of 5HT1A receptors at the second challenge in the study by Shiah et al30 may have been caused by the challenge conducted only 1 week before; Gilmore et al50 reported significant blunting of the prolactin response to a clomipramine rechallenge at 2 weeks but not at 4 weeks. Furthermore, the potent endocrine effects of nicotine51–53 may have affected the findings of Shiah et al,30 who included smokers as subjects. Finally, Shiah et al30 employed a much shorter period of starvation before the ipsapirone challenge tests than we did and did not measure plasma ipsapirone levels. Thus, although we can be confident that our 2 ipsapirone challenges were equivalent, it is possible that any changes in effect on temperature in the study by Shiah et al30 may have been the consequence of differences in plasma ipsapirone levels during the 2 challenges. Valproate has been reported to cause hypothermia,54 an outcome which could obviously interfere with the interpretation of the effect of ipsapirone on body temperature. Although there was no evidence of valproate-induced hypothermia in our study, it is of note that in the Shiah et al30 study, there was a trend toward lower baseline body temperature at the ipsapirone challenge during valproate treatment in comparison with the baseline measure before valproate.
Because ipsapirone is a specific 5-HT1A receptor probe, our findings do not rule out the possibility that valproate treatment affects other 5-HT receptor subtypes. For example, using a nonspecific 5-HT probe (L-5-hydroxytryptophan), Maes et al10 found that plasma cortisol responses were significantly higher in manic patients after receiving valproate for 3 weeks than before treatment. A putative increase in 5-HT neurotransmission in the presence of valproate10 may involve elements of the 5-HT system other than changes in the 5-HT1A receptor investigated in this study. Valproate may also have direct or indirect effects on 5-HT1A receptors other than the regulatory effects we examined. Ichikawa et al55 concluded that valproate interacts with 5-HT1A receptors to produce increased DA in the prefontal cortex of the rat brain because such increases were blocked by a 5-HT1A antagonist. Because there is no evidence of appreciable affinity of valproate for 5-HT1A receptors or other monoamine receptor subtypes, however, Ichihawa et al55 suggest that valproate stimulates the 5-HT1A receptors in an indirect fashion.
Valproate and dopamine receptors
Apomorphine effectively induced release of GH and inhibition of prolactin,39 but valproate treatment failed to change these neuroendocrine responses to apomorphine. Because apomorphine is a nonspecific DA receptor probe, we can conclude that up to 625 mg/d of valproate has no apparent effect on DA receptors.
Few studies using animal or human subjects have been conducted to examine the dopaminergic effects of valproate. Evidence from studies examining the effects of lithium and carbamazepine on DA function suggests that dopaminergic systems may be involved in the mechanism of action of mood stabilizers. Treatment with lithium in patients with psychiatric disorders does not appear to influence the GH response to apomorphine.56,57 However, lithium treatment has been shown to block the apomorphine-induced decrease in prolactin in the rat58,59 and cause a small reduction in the prolactin response to apomorphine in patients with affective illness.57 Carbamazepine treatment may also alter brain DA function, as found by Elphick et al,41 who demonstrated an enhanced GH response to apomorphine after short-term treatment with carbamazepine in healthy male participants. Despite the apparent lack of effect of valproate on DA receptor-mediated hypothalamic or pituitary responses, it is possible that valproate affects DA receptors in other brain regions or other aspects of DA function, including synthesis, release or reuptake. For instance, clozapine, reported as an effective mood stabilizer, increases DA release in the medial prefrontal cortex via the 5-HT1A receptor.60 Carbamazepine and valproate also appear to share this mechanism of DA release, as they too have been shown to increase prefrontal DA levels.55
Our findings suggest that short-term treatment with valproate at a daily dose of 625 mg fails to affect 5-HT1A receptor mediated changes in 5-HT neurotransmission; in addition, it does not appear to affect DA neurotransmission via change in DA receptor function in hypothalamic and pituitary regions. Given the limited and contradictory reports available on the effects of valproate on 5-HT and DA receptors in humans, further investigations with other neuroendocrine challenges are needed to elucidate the time- and dose-related effects of valproate on 5-HT, DA and other neurotransmitter systems.
Acknowledgements
We thank the Royal Ottawa Hospital Foundation, the University of Ottawa Medical Research Fund and the Kingston Psychiatric Hospital Research Fund for providing the funding for this study, and Abbott Laboratories, Ltd., and Bayer for supplying valproate and ipsapirone, respectively. We thank all of those who participated in the study, as well as those who helped in its completion: Ms. L. DiPietro, Dr. R. Kesteven and Mr. S. Layton.
Footnotes
Medical subject headings: apomorphine; dopamine; hypothermia; receptors, serotonin; serotonin; serotonin agonists; valproic acid.
Competing interests: None declared for Drs. Delva, Franklin, Al-Said, Merali or Lawson; Ms. Brooks or Ms. Hawken. Dr. Ravindran has received educational grants, speaker fees and research grants from a number pharmaceutical companies, but not from the manufacturer of valproate, Abbott Laboratories.
- Received October 3, 2001.
- Revision received March 7, 2002.
- Accepted May 21, 2002.