Abstract
Objective: The plasticity of the startle reflex, including prepulse inhibition (PPI) and habituation, provides operational measures of information processing that are abnormal in several neuropsychiatric disorders characterized by deficits in suppression or inhibition of intrusive or irrelevant stimuli. Clinically, patients with panic disorder (PD) have been described as having difficulties in the inhibition of their response to sensory and cognitive events. Because such difficulties may be the result of failures in early stages of information processing, we hypothesized that startle reactivity, PPI and habituation are deficient in unmedicated patients with PD. Moreover, we tested whether there was a relation between startle reflex measures and dysfunctional cognition.
Methods: Fourteen unmedicated patients with PD (7 men, 7 women) and 28 healthy comparison subjects (14 men, 14 women) were recruited. Acoustic startle reactivity, habituation and PPI (30-ms, 60-ms, 120-ms, 240-ms and 2000-ms interstimulus intervals) were assessed in the patients with PD and the age-matched and sex-matched healthy controls. These data for unmedicated patients with PD were compared with those for 24 medicated patients with PD. Moreover, dysfunctional cognition in patients with PD was measured using the Body Sensations Questionnaire.
Results: Unmedicated patients with PD exhibited increased startle reactivity, reduced habituation and significantly reduced PPI in the 30-ms, 60-ms, 120-ms and 240-ms prepulse conditions. Furthermore, in unmedicated patients with PD, increased startle response and decreased habituation were correlated significantly with higher cognitive dysfunction scores, but this was not the case for PPI.
Conclusions: These data indicate that the early stages of sensory information processing are abnormal in patients with PD in the absence of medication. The observed deficits in PPI and habituation could reflect a more generalized difficulty in suppressing or gating information in PD. The correlation between cognitive symptoms and higher startle response and deficient habituation supports the hypothesis that subjects with PD have abnormalities in the early stages of information processing that lead to a cascade of downstream effects on cognition.
Introduction
Panic disorder (PD) is a common and often chronic illness that initially causes unprovoked, intense episodes of fear. PD is associated with an impaired quality of life similar to or greater than that associated with major depression.1 The main symptoms of PD are recurrent anxiety attacks accompanied by physiologic symptoms, in particular, palpitations, sweating, trembling and shortness of breath.2 According to cognitive models of psychopathology, anxiety disorders such as PD are characterized by various kinds of cognitive biases.3,4 Patients with PD are very sensitive to somatic sensations and show fear of specific physical anxiety symptoms. Moreover, patients with PD misinterpret somatic sensations and exhibit dysfunctional cognition such as widespread catastrophic thinking.4–6 These symptoms suggest abnormalities of sensory and cognitive information processing.
The startle response is defined as “an immediate reflex response to sudden, intense stimulation” measured by the eye-blink reflex component of the startle response.7 Previous studies have reported that the acoustic startle reflex, a rapid escape response elicited by a sudden and intense auditory stimulus,8 is increased in patients with anxiety disorders9 and in healthy subjects viewing aversive pictures.10 An increased startle response is also found in individuals with simple phobia11 and in Vietnam veterans with posttraumatic stress disorder (PTSD).12–14 Other studies did not find an increased startle response in healthy subjects with high state/trait anxiety,15 PD16 or Vietnam veterans with PTSD in the baseline condition.17
Features of the plasticity of the startle reflex, such as prepulse inhibition (PPI), have been widely used as neurophysiologic measures of the early stages of information processing. PPI is the normal unlearned suppression of the startle reflex when the startle-eliciting stimulus is immediately preceded by a weak acoustic stimulus. PPI of the startle reflex represents a general ability to inhibit external (auditory, visual, tactile) and internal (thoughts, impulses) stimuli. In recent years, PPI has been the focus of interest in a range of neuropsychiatric disorders in which processes of sensory or cognitive function are thought to be impaired. Disorders with PPI deficits “are characterized by a loss of the normal ability to suppress or gate irrelevant sensory, motor or cognitive information. This loss of ‘gating’ may be experienced as intrusive thoughts, sensory information or adventitious movements or behaviours”(p. 177).18 A previous study in medicated patients with PD showed that PPI is reduced.19 This result supports the hypothesis that PPI deficits could reflect an inability to suppress or gate information in PD. In the clinical field of anxiety disorders, PPI studies in PTSD have provided conflicting results.12,17
Another form of the plasticity of the startle reflex is habituation. Habituation, defined simply as a decreased response to repeated stimulation, is considered to be the simplest form of learning and is essential for the maintenance of selective attention. 20 Startle habituation is deficient in patients with schizophrenia,21,22 which is consistent with an attentional or cognitively mediated deficit in information processing. Similarly, deficient habituation could lead to the kind of excessive response to sensory inputs observed in PD.
Unmedicated patients with PD have not yet been examined regarding PPI and habituation of the startle reflex, as far as we know. We investigated startle reactivity, PPI and habituation in unmedicated patients with PD to test the general hypothesis that abnormalities in the processing of sensory information may exist in this population. Our second aim was to test whether unmedicated patients with PD differ from medicated patients with PD in the startle and PPI measures. A third purpose of the present study was to test the relation between measures of information processing and cognitive dysfunction in patients with PD.
Methods
Fourteen unmedicated patients with diagnosis of PD with or without agoraphobia according to the criteria of the International Statistical Classification of Diseases and Related Health Problems, 10th revision23 (ICD-10, F40.01, F41.0) and the Diagnostic and Statistical Manual of Mental Disorders, fourth edition2 (DSM-IV, 300.01, 300.21) were recruited. The patients’ conditions were diagnosed according ICD-10 and DSM-IV diagnostic criteria based on an individual semistructured psychiatric interview by an experienced clinician using the German version of the international diagnostic checklists.24 Most of the patients were outpatients. Twenty-eight healthy comparison subjects with no personal history of psychiatric
disorder were hospital employees or were recruited through local advertisements. Healthy comparison subjects (controls) and patients reported no history of major medical disturbances. The patient and control groups were age-matched and sex-matched: the patient group consisted of 7 men and 7 women whose mean age was 34 (standard deviation [SD] 10, range 20–50) years; the comparison group consisted of 14 men and 14 women whose mean age was 35 (SD 10, range 21–52) years. All subjects, whether patients or controls, were unmedicated. If patients had received medication previously, they had now been drug free for at least 5 half-lives; most patients had never been medicated or had taken no medication for several months before testing. Information about medication and the use of psychoactive substances was obtained using a partial standardized questionnaire. Subjects were asked not to use nicotine and caffeine before or during the testing. Anxiety symptoms were rated in patients with PD using the Hamilton Rating Scale for Anxiety (HAM-A25). Dysfunctional cognition was operationalized using the Body Sensations Questionnaire (BSQ26), which measures cognitive misinterpretation of somatic symptoms. Because of difficulties with the German language, 1 patient was not able to fill in the questionnaires.
A second question considered in the this study involved the comparison of medicated and unmedicated patients with PD. The data from most of the medicated patients with PD used for this comparison have been presented previously.19 The design of the startle paradigm and the experimental setting and procedures were identical to those described in the previous study,19 although the measurements of dysfunctional cognition were not used previously. The medicated patients with PD consisted of 10 men and 14 women whose mean age was 36 (SD 10, range 19–61) years. Most of these patients were being treated with selective serotonin reuptake inhibitors (SSRIs) (n = 14), some with tricyclic antidepressants (TCAs) (n = 5), 2 with both SSRIs and TCAs, 1 with a combined noradrenaline–serotonin reuptake inhibitor, 1 with a low potency neuroleptic and 1 with a phytotherapeutic substance. Some of the data for the healthy controls were previously used for an analysis of age and sex in healthy control subjects27 and the aforementioned PD paper.19
After a complete description of the study was given to the participants, written informed consent was obtained. The study protocol and consent forms were reviewed and approved by the Ethics Committee of the Psychiatric Services of Aargau Canton, Switzerland.
Startle response measurement
Participants were seated comfortably in an armchair and were instructed to keep their eyes open. The eyeblink component of the acoustic startle response was measured using an electromyographic (EMG) startle system (EMG-SR-LAB, San Diego Instruments, San Diego, Calif.). The registration parameters have been described in detail elsewhere.22 Two silver/silver-chloride electrodes (11 mm) were placed below the right eye over the orbicularis oculi muscle, and a ground electrode (San Diego Instruments) was placed behind the right ear. Electrodes were attached with electrode washers (20 mm). All electrode resistances were less than 5 kΩ. The system recorded 250 1-ms epochs, starting with the onset of the startle stimulus. In addition, EMG activity was bandpass filtered (100–500 Hz). A 50-Hz notch filter was also used to eliminate 50-Hz interference. A square wave calibrator established sensitivity to be 4.7 μV per digital unit. Background noise and stimuli were presented through headphones (TDH-39P, Maico, Minneapolis, Minn.). Each session began with a 5-minute acclimatization period of 70-dB background broadband noise that continued throughout the session. Sound levels were calibrated monthly by using a continuous tone and sound-level meter with a coupler in an artificial ear and were found to be stable. A mixture of pulse-alone and prepulse-and-pulse trials was used to investigate startle, habituation and PPI. This paradigm was designed to measure both PPI and habituation in 1 session to minimize intersession differences and interactions that occur when 2 different sessions are used sequentially.22 The session comprised 52 trials consisting of 2 conditions: (1) a 115-dB pulse-alone condition that lasted 40 ms; and (2) the same pulse preceded by a 86-dB (16 dB above background) prepulse (pp) lasting 20 ms at 30 ms, 60 ms, 120 ms, 240 ms or 2000 ms (pp30, pp60, pp120, pp240, pp2000, respectively). The first and last blocks of a session consisted of 6 pulse-alone trials that were used for the calculation of habituation, but not for the calculation of PPI. The middle block of 40 trials consisted of 10 pulse-alone trials and 6 of each of the prepulse trials (pp30, pp60, pp120, pp240, pp2000) presented in a pseudo-random order. The entire test session lasted about 18 minutes. All recordings were screened to exclude spontaneous eyeblink activity before data analysis, with about 5% of trials being excluded based on previously described criteria.22
The startle measures examined were as follows: (1) startle magnitudes across blocks 1–3, assessing both startle reactivity and habituation uncorrected for differences in response magnitudes; (2) habituation corrected for differences in startle magnitudes, expressed as the percentage of habituation, according to the formula (1-[mean startle magnitude for block 1/mean startle magnitude for block 3]) × 100; (3) percentage change (%) for PPI and prepulse facilitation, according to the formula (1-[mean startle magnitude on prepulse {pp30, pp60, pp120, pp240, or pp2000} trials]/mean startle magnitude on pulse-alone trials [block 2] × 100) × 100; and (4) peak latency, the latency time between stimulus onset and peak magnitude for each trial type (block 2, pp30, pp60, pp120, pp240 and pp2000). No latency values were calculated for trials in which an identifiable blink response was not found. Peak rather than onset latencies were calculated because, unlike onset latencies, there is no confounding of peak latencies by changes in reflex magnitude.28
Statistical analysis
Data were analyzed using repeated-measures analysis of variance (ANOVA), with trial type and session as within-subject factors and group (unmedicated patients with PD v. healthy control subjects, and unmedicated v. medicated patients with PD v. healthy control subjects) as a between-subject factor. Following significant interaction effects, post hoc analyses were conducted using an alpha level of p < 0.05. Spearman rank correlations were calculated to examine the relation between startle measurements and measures of dysfunctional cognition rated by BSQ.26 A comparison of the clinical characteristics of medicated and unmedicated patients with PD was performed with an independent-samples t test. The statistical analyses were performed using STATISTICA/w (StatSoft).
Results
Findings in unmedicated patients with PD compared with healthy controls
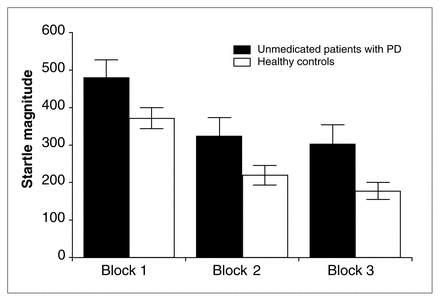
The unmedicated patients with PD showed moderate symptoms of anxiety (mean total HAM-A score 18.2 [SD 5.8]). The measures of cognition regarding fear of somatic symptoms were also increased (mean BSQ score 2.3 [SD 0.7]). Patients with PD and controls showed differences in startle reactivity (startle magnitude in block 1: F1,40 = 4.1, p < 0.05), with higher startle response in patients with PD (Fig. 1). The analysis of pulse-alone magnitudes across the entire session (blocks 1–3) showed significant differences between patients and controls (Fig. 1; F1,40 = 5.8, p < 0.02). There was a significant block effect (F2,80 = 75.1, p < 0.001), with lower startle magnitudes in the last block than in earlier blocks, reflecting the phenomenon of habituation (Fig. 1). There was no group × block interaction.
Patients with unmedicated panic disorder (n = 14) showed significantly higher startle magnitudes in all 3 blocks of the test session compared with healthy control subjects (n = 28).
Patients with PD exhibited a significantly lower percentage of habituation than controls (F1,40 = 4.2, p < 0.05).
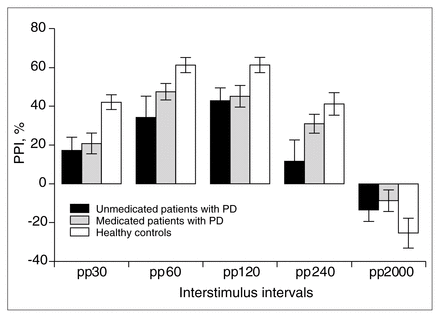
The main effect of group (patients v. controls) on PPI was significant (F1,40 = 7.2, p < 0.01.), and there was a significant group × condition interaction (F4,160 = 4.8, p < 0.001). Post hoc ANOVA revealed significant differences in the pp30 (F1,40 = 12.0, p < 0.001), pp60 (F1,40 = 8.1, p < 0.007), pp120 (F1,40 = 6.5, p < 0.02) and pp240 conditions (F1,40 = 6.9, p < 0.01).
No significant (patients v. controls) effects or interaction effects were found on peak latency (middle block, pp30 to pp2000).
In the patient group, there was no significant correlation between cognitive processing and PPI values. There was a significant correlation between the measure of cognitive misinterpretation of somatic symptoms26 and percentage of habituation (Spearman R = −0.73, t = −3.6; p < 0.005; Fig. 2). Furthermore, BSQ values were correlated positively with startle magnitude in the middle (Spearman R = 0.62, t = 2.6; p < 0.03) and the last blocks (Spearman R = 0.73, t = 3.5; p < 0.005).
Spearman rank correlation between measures of cognitive misinterpretation of somatic symptoms evaluated using the Body Sensations Questionnaire (BSQ) and habituation in unmedicated patients with panic disorder (n = 13). Patients with more deficient habituation showed higher scores for dysfunctional cognition on the BSQ.
Findings in unmedicated patients with PD compared with medicated patients with PD compared with healthy controls
The medicated patients with PD showed marked symptoms of anxiety (mean total HAM-A score 26.2 [SD 10.0]). The HAM-A score differed significantly between the unmedicated and medicated patient groups (total HAM-A score: t32 = −2.4, p < 0.03). The startle magnitude differed between groups (F2,63 = 4.7, p < 0.01), but there was no interaction with blocks. A post hoc comparison between medicated and unmedicated patients with PD revealed no significant differences.
We found a group effect on habituation (F2,63 = 4.2, p < 0.02), with less habituation in both patient groups compared with controls. The post hoc comparison showed no difference between the medicated and unmedicated groups of patients with PD.
The main effect of group on PPI (F2,63 = 4.6, p < 0.01) and the group × condition interaction was significant (F8,252 = 3.8, p < 0.001; Fig. 3). The post hoc analysis showed a statistical trend of less PPI in the pp240 condition in unmedicated compared with medicated patients (F1,63 = 3.4, p < 0.06).
Prepulse inhibition (PPI) in medicated (n = 24) versus unmedicated patients with panic disorder (n = 14) versus healthy control subjects (n = 28). Both groups with panic disorder showed reduced PPI; the PPI in the unmedicated patient group was more deficient in the 240-ms prepulse condition.
Discussion
Our results support the hypothesis that unmedicated patients with PD exhibit increased startle reactivity and deficits in both PPI and habituation of the startle response. Some evidence indicated that cognitive misinterpretation of somatic symptoms is correlated with higher startle reactivity and reduced habituation, but not with PPI.
The unmedicated patients with PD showed increased startle reactivity compared with healthy controls. This finding confirms and extends previous reports that patients with PD and patients with PTSD exhibit increased startle response magnitudes during baseline or stressful conditions.11,12,16,19 Most of the studies have investigated patients with PD in the fear-conditioned startle paradigm: after Pavlovian fear conditioning, subjects showed an increased startle response.29,30 Patients with PD,16 healthy subjects with high trait anxiety15 and adolescents with a family history of anxiety disorders exhibit increased startle response in the fear-conditioned startle paradigm. 31 In addition, exaggerated startle constitutes a symptom of anxiety disorders such as PTSD and generalized anxiety disorder (DSM-IV2). These data support the hypotheses that increased startle reactivity may be a common symptom and a sensitive measure in PD.
Confirming the results of a previous study in medicated patients with PD,19 PPI was markedly reduced in the 30-ms, 60-ms, 120-ms and 240-ms prepulse conditions in patients with PD, in the absence of significant changes in peak latency. This result may reflect a link between PPI deficits and the psychopathology of PD. The inhibition of the startle response when the startle stimulus is preceded by a prepulse (PPI) protects the early stages of stimulus processing in humans.32 When PPI and gating functions are reduced, it is conceivable that patients with PD are in a heightened state of sensory overload, which could promote the development of panic symptoms. These findings of deficient information processing in patients with PD are in accord with studies using measures of evoked potentials. Both auditory evoked potentials33 and brain-stem34 evoked potentials showed higher amplitudes in patients with PD, differences that were discussed in relation to the pathophysiology and neurochemistry of this disorder.
Animal studies provide evidence that PPI is regulated by dopaminergic substrates. PPI is reduced by manipulations that directly decrease frontal cortical dopamine activity35 and by manipulations that directly increase dopamine activity in subcortical mesolimbic terminal fields.36,37 In addition, the ability of antipsychotics to reverse PPI deficits in dopamine agonist–treated rats correlates significantly with their clinical antipsychotic potency.37,38 Moreover, several lines of evidence suggest that dopamine might be involved in anxiety states, including the possibility of dopaminergic overactivity in PD.39,40 Therefore, future studies could examine the effect of modulating dopaminergic activity to determine whether PPI deficits in subjects with PD are modified and, thus, related to an altered dopaminergic tone.
There is some evidence that other neurotransmitter systems such as corticotropin-releasing factor (CRF) appear to be abnormally regulated in PD.41 CRF receptors are expressed in the cortex, striatum, hippocampus, nucleus accumbens and the basolateral nucleus of the amygdala,42,43 brain areas known to be crucial for control of PPI.44 Interestingly, CRF increases the acoustic startle reflex via its action in the extended amygdala and decreases PPI when given to rats or mice.45,46 Therefore, future studies could investigate whether the plasticity of the startle reflex is modulated by CRF and whether antagonists of these neurotransmitters may prove to be beneficial anxiolytics in patients with PD.
The anatomical basis of deficient PPI in PD is unclear and remains to be determined. Preclinical studies suggest that PPI is modulated by cortico-striato-pallido-pontine circuits9,42 that include brain regions that are implicated in a “fear network” in PD, in particular, the amygdala, which is a critical target for modulating dopaminergic innervation.47 Animal studies have provided strong evidence for involvement of the amygdala in the production of fear and anxiety.48–50 Various brain structures, including the locus coeruleus and the raphe nuclei, 51,52 the temporal lobe and limbic structures46,53–55 and the amygdala,56 are implicated in theories examining the pathogenesis of PD. Therefore, it is conceivable that the amygdalar region may be abnormally sensitive in patients with PD.
Patients with PD exhibited a reduction in habituation of the startle reflex compared with healthy controls. Deficits in habituation of the startle reflex were first shown in patients with schizophrenia and have been discussed in relation to sensory overload in the schizophrenia patient group.21 Whereas PPI is considered to reflect pre-attentive processing of stimuli, which is largely independent of cognitive intervention, normal habituation is necessary for individuals to learn to discriminate relevant from irrelevant stimuli and involves clearly cognitive assessments. One possible clinical implication is that deficits in habituation lead to an exaggerated responsiveness to anxiety-eliciting stimuli, which makes subjects with PD more sensitive to threatening information. To our knowledge, this is the first study that has reported a significant correlation between decreased habituation and cognitive symptoms in unmedicated patients with PD. The relation between habituation and the abnormal cognition characteristic of PD, involving concerns regarding somatic symptoms and catastrophic misinterpretations of body sensations, 4,6 supports the hypothesis that patients with PD have deficits in the early stages of information processing, which lead to a cascade of downstream effects on cognition. Deficits in habituation could also lead to a cognitive overload that could overwhelm the information-processing resources of these patients. Generally, it is conceivable that informationprocessing disturbances such as impaired habituation could be viewed as central elements of the cognitive deficits that characterize PD.
The study showed that PPI, habituation and startle reactivity differences were found in both medicated and unmedicated patients. The PPI in the unmedicated PD group was more deficient than in medicated PD patients, indicating that medical treatment could improve PPI. This difference is striking given the trend for higher symptom scores in the medicated group than in the unmedicated group. A within-subjects longitudinal treatment study would provide more information about the medication effect on PPI in patients with PD.
These results support the hypothesis that unmedicated patients with PD exhibit increased startle reactivity as well as deficits in PPI and habituation of the startle response. Cognitive dysfunctions such as cognitive misinterpretation of somatic symptoms are correlated with the startle response and habituation, but not with PPI. Further investigations are needed to confirm and extend these findings of increased startle reactivity, deficient PPI and reduction in habituation in patients with PD. Future studies are warranted to investigate the effects of modulating dopaminergic activity on plasticity of the startle response in subjects with PD and to examine startle habituation in PD.
Acknowledgements
This study has been supported by a grant from the Stiftung für Forschung im Gesundheitswesen des Kantons Aargau given to S.L.
Footnotes
Medical subject headings: cognition; habituation (psychophysiology); panic disorder; startle reaction.
Competing interests: None declared for Drs. Ludewig, Ramseier, Vollenweider, Rechsteiner and Cattapan-Ludewig. Dr. Geyer has an equity interest in San Diego Instruments.
- Received September 19, 2003.
- Revision received January 20, 2004.
- Accepted January 27, 2004.