Abstract
Objective: In a previous study, we demonstrated that amygdala reactivity to masked negative facial emotions predicts negative judgmental bias in healthy subjects. In the present study, we extended the paradigm to a sample of 35 inpatients suffering from depression to investigate the effect of amygdala reactivity on automatic negative judgmental bias and clinical characteristics in depression.
Methods: Amygdala activity was recorded in response to masked displays of angry, sad and happy facial expressions by means of functional magnetic resonance imaging at 3 T. In a subsequent experiment, the patients performed an affective priming task that characterizes automatic emotion processing by investigating the biasing effect of subliminally presented emotional faces on evaluative ratings to subsequently presented neutral stimuli.
Results: Significant associations between (right) amygdala reactivity and automatic negative judgmental bias were replicated in our patient sample (r = −0.59, p < 0.001). Further, negatively biased evaluative processing was associated with severity and longer course of illness (r = −0.57, p = 0.001).
Conclusion: Amygdala hyperactivity is a neural substrate of negatively biased automatic emotion processing that could be a determinant for a more severe disease course.
Introduction
Major depressive disorder is the leading global cause of years of life lived with disability1 and ranks among the top 3 health conditions concerning burden of disease.2 Cognitive theories suggest that the onset and maintenance of depression is affected by abnormal emotion processing in terms of negative cognitive biases.3 A large body of research has made use of paradigms derived from experimental cognitive psychology and confirmed several predictions.4 Negative cognitive biases in depression have already been identified at automatic stages of emotion processing,5 and strong automatic negative judgmental biases for facial expressions were shown to be a predictive factor for weak therapy response in a longitudinal study.6 However, the neural substrates of automatic judgmental biases remain to be clarified.
During the last decade, an increasing number of studies have investigated the neural correlates of major depression by means of functional MRI (fMRI). Amygdala hyperactivity has been demonstrated in patients with acute depression, compared with control subjects at rest,7 in expectation of negative pictures8 and in response to verbal stimuli9 and emotional faces10; this was shown to resolve after antidepressant therapy.11 Therefore, amygdala hyperactivity, probably causing negatively biased emotion processing,12,13 has been implicated in the pathogenesis of major depression.
Neurobiological theories of emotion processing suggest 2 separate neural pathways, one operating at the conscious level and another operating on an automatic, implicit level.14–16 Studies investigating the neural substrates of automatic emotion processing usually employ backward-masking techniques in which emotional stimuli are presented briefly (< 40 ms) and masked to prevent conscious perception.17 Backward-masking was used in several neuroimaging studies to demonstrate that the amygdala is critically involved in the automatic processing of negative facial expressions.18–20 The amygdala is thought to be particularly engaged during the rapid processing of negative stimuli, guiding attention resources toward possible sources of danger.21 Thus we speculated that amygdala hyperactivity might be the neural substrate of automatic judgmental bias in depression.
In a previous study, we investigated the effect of amygdala reactivity on automatic appraisal processes in a sample of healthy subjects.22 Combined fMRI and experimental psychology were employed to link amygdala activity to negative judgmental bias as measured by a cognitive paradigm in the same patients. Amygdala activity was measured in response to backward-masked displays of positive and negative facial expressions. In a subsequent experimental test session, the masked affective priming paradigm of Murphy and Zajonc23 was administered outside the scanner to assess automatic affective processing characteristics. In this task, automatic appraisal processes are characterized by investigating the biasing influence of masked emotional prime faces on evaluative ratings of subsequently presented neutral target stimuli. As expected, it could be demonstrated that amygdala responses to masked negative faces predict negative judgmental biases elicited by corresponding facial expressions in the affective priming task. No such correlation was found for happy facial expressions, although a significant positive bias based on masked happy faces was found.
In the present study, we sought to replicate and extend these findings to a larger sample of patients suffering from major depression. We predicted that strong amygdala responses to masked negative faces are associated with automatic negative biases and a long duration and severity of illness (as indicated by lifetime hospitalization or number of episodes) but not necessarily with current symptoms. Possible confounding variables such as trait anxiety and awareness were taken into account. Etkin and colleagues demonstrated that trait anxiety may be positively associated with amygdala response to masked negative (fearful) facial expressions.24 Pessoa and colleagues reported that amygdala activity in response to masked faces might depend on the success of the masking procedure.25
Methods
Subjects
Thirty-five inpatients (24 women and 11 men) diagnosed by means of the Structured Clinical Interview for DSM-IV26 participated in the study. All patients suffered from acute major depression and all were on antidepressant medication. Exclusion criteria were any history of mania or hypomania, neurologic illnesses, benzodiazepine treatment, former electroconvulsive therapy, age of 60 years and older, and the usual MRI contraindications. All subjects had normal or corrected-to-normal vision. The State-Trait Anxiety Inventory (STAI), a measure of anxious emotional and cognitive reactions, was administered in its trait form.27 Depression severity was assessed with the Beck Depression Inventory (BDI)28,29 and the Hamilton Depression Rating Scale (HAMD)30,31 (see Table 1 for details). The experiments were conducted in accordance with the Declaration of Helsinki. The ethics committee at the University of Münster approved the study. After the nature of the procedures had been fully explained, informed written consent was obtained from all patients before the study commenced.
Sociodemographic, clinical and affective characteristics of the depression patients included in the final analysis (n = 28)
Facial emotion presentation in the fMRI session
Details of the fMRI stimulus presentation, data analysis and the affective priming paradigm task have been described previously.22 Briefly, facial stimuli consisted of sad, angry, happy and neutral expressions.32 Subjects were presented with alternating 30-second epochs of 1 of the 4 facial expressions or a no-face stimulus (a grey rectangle). Emotional faces were shown twice per second for 33 milliseconds followed by a neutral face mask for 467 milliseconds. Any emotional face was always masked by a neutral face of the same individual. The order of epochs was counterbalanced across subjects. Each face epoch was presented twice and was preceded by a no-face epoch, resulting in an overall presentation time of 8 minutes. Images were displayed via projection to the rear end of the scanner (Sharp XG-PC10XE with additional HF shielding).
fMRI methods
T2-weighted functional data were acquired on a 3 Tesla scanner (Gyroscan Intera 3.0T, Philips Medical Systems, Best, The Netherlands) by using a single shot echoplanar sequence with parameters selected according to suggestions made by Robinson and colleagues.33 Volumes consisting of 25 axial slices were acquired (matrix 128 × 128, resolution 1.75 × 1.75 × 3.5 mm, repetition time = 3 s, echo time = 30 ms, flip angle = 90°) 160 times in blocked design and 10 times per condition. To optimize the following normalization procedures, the same sequence parameters were used to cover the whole brain with 43 slices. Functional imaging data were motion corrected, spatially normalized to standard Montreal Neurological Institute space and smoothed (Gaussian kernel, 6 mm full width at half maximum) with SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Statistical analysis was performed by modelling the different conditions (angry, sad, happy, neutral and no face) as variables within the context of the general linear model (modelled with a standard hemodynamic response function). On a single-subject level, we calculated contrast images comparing each masked emotion face epoch (happy, angry, sad and overall negative) with the neutral face baseline.
Activity within the amygdala in response to negative faces was correlated with judgmental bias (the “bias scores”) elicited by negative faces in the affective priming task. We selected a voxel-wise region of interest (ROI) approach on the basis of our a priori hypothesis concerning amygdala activity, particularly in the negative face condition. A mask for bilateral amygdala was created with WFU PickAtlas34 and the amygdala was defined according to Tzourio-Mazoyer and colleagues.35 We conducted the analysis at p < 0.001, with a minimum cluster size (k) of 5 voxels. Significant clusters are reported corrected for the amygdala volume (p < 0.05). In addition, we conducted a whole-brain analysis at p < 0.05, corrected for multiple comparisons across the entire brain.
We further investigated the effect of different emotional qualities, particularly happy faces. Amygdala voxel values were extracted, summarized by mean and tested among the different conditions with the MarsBaR toolbox.36 Pearson’s product-moment correlation coefficients were calculated between the averaged amygdala contrast values (left and right) and bias scores for the corresponding emotion in the affective priming task (for each emotion quality separately). To explore a possible confounding effect of trait anxiety (STAI trait score) and detection task performance, we also conducted partial correlations controlling these variables.
Affective priming task
Identical face stimuli were used for the fMRI presentation and the affective priming task. Ten pictures of neutral facial expressions served as target stimuli. Prime stimuli were pictures of the same actors showing happy, sad, angry or neutral facial expressions. Each of the 40 trials had the following routine: after a fixation cross lasting for 500 milliseconds, a prime face was presented for 33 milliseconds and directly followed by a neutral target face for 333 milliseconds. Each target picture was presented 4 times during the experiment, each time primed by a different facial emotion. The presentation order was randomized for each subject separately. The subjects were instructed to view a series of faces and evaluate the expression on a 7-point scale ranging from −3 to +3 by pressing a button on the keyboard. They were told that the faces had slight differences concerning their affective expressions. For each emotion (happy, sad, angry and overall negative), we computed a bias score by subtracting mean evaluative ratings for neutral target faces primed by neutral faces from mean evaluative ratings for neutral target faces primed by emotional faces. A negative bias score (e.g., for angry faces) indicates that the subjects rated the neutral targets more negatively if they were primed by angry faces, compared with neutral targets primed by neutral faces, whereas a positive bias score indicates a positive evaluative shift. If the subjects were unaware of the prime faces, a bias score should reflect automatic evaluative processes.
Detection task
The detection task was designed to assess objective awareness of the prime faces. After the affective priming task, the subjects were informed about the presence of emotional prime faces while in the MRI scanner and during the affective priming task. Then they were asked for subjective awareness of the emotional prime faces. The detection task was administered in the following manner: subjects saw the (prime–target) stimulus sequences of the affective priming task and were asked to indicate by button press which emotion quality was displayed briefly as prime (happy, sad, angry or neutral). In each trial of the detection task, a prime face was presented for 33 milliseconds preceded by a fixation cross lasting 500 milliseconds and followed immediately by a neutral target face for 333 milliseconds. The stimulus presentation procedure was identical to the affective priming task. The chance level for correct answers was 25%. According to a 1-tailed binominal test, scores of 15 hits (37.5% hit rate) and above are significantly above chance level.
Results
Detection task
No patient reported having seen any features of briefly presented emotional faces in the MRI scanner or during the affective priming task. However, 7 patients performed above chance (> 14 hits) in the detection task. These patients were considered “aware” and consequently their data were removed from further analysis. The remaining 28 patients performed at or below chance level (mean hit rate 25.9%; range 15%–35%; i.e., 6–14 hits).
Affective priming task
Evaluative ratings of the remaining patients were entered into a repeated-measures analysis of variance with prime (happy, sad, angry or neutral) as the within-subjects factor. No significant prime effect on evaluative ratings was observed, F3, 81 = 1.7, p = 0.18.
Amygdala activity in response to masked emotional stimuli
For the sake of comparability with most of the previous studies on amygdala activity that used a fixation cross baseline condition,10,20,37,38 we conducted an ROI-analysis of amygdala activity in response to emotional faces compared with the no-face baseline (at p < 0.05, corrected). Significant clusters within the left and right amygdala were detected for masked sad faces (t27 = 5.41, pcorrected = 0.002, x = −22, y = −6, z = −16, k = 36; t27 = 5.95, pcorrected = 0.001, x = 22, y = −4, z = −18; k = 58) and masked angry faces (t27 = 4.22, pcorrected = 0.02, x = −20, y = −6, z = −14, k = 10; t27 = 5.25, pcorrected = 0.001, x = 32, y = −4, z = −20, k = 49). However, presentation of happy faces was associated with activations only in the right amygdala (t27 = 6.03, pcorrected = 0.006, x = 32, y = −6, z = −14, k = 24).
In comparison with the neutral-face baseline, no significant amygdala activation was found when we used this conservative threshold. We repeated the analysis in accordance with Nomura and colleagues.38 and Killgore and Yurgelun-Todd,37 who used a more lenient threshold of p < 0.05, uncorrected. Significant voxels were detected within left and right amygdala in response to sad-neutral (t27 = 1.82, puncorrected = 0.039, x = −20, y = −4, z = −16, k = 10; t27 = 2.14, puncorrected = 0.02, x = 36, y = 0, z = −26; k = 13) and angry-neutral (t27 = 1.74, puncorrected = 0.046, x = −30, y = −2, z = −26, k = 1; t27 = 2.45, puncorrected = 0.01, x = 32, y = −4, z = −20; k = 14). No activation by masked happy faces could be detected in comparison with the neutral face baseline. No effect of sex in response to any emotional quality was observed with respect to bias scores or amygdala activity (all p > 0.20).
Correlation analysis of neural responses with affective priming
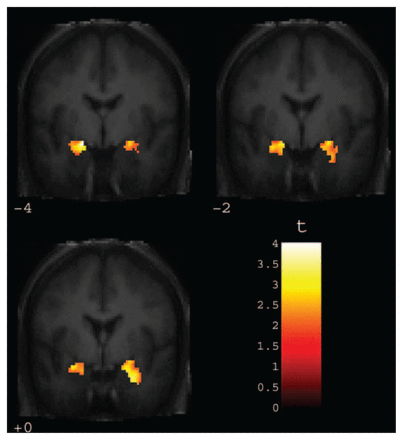
Two clusters were detected in bilateral amygdala that correlated significantly with negative bias scores (t26 = 4.01, pcorrected = 0.014, x = −18, y = −4, z = −12, k = 13; t26 = 3.82, pcorrected = 0.022, x = 22, y = 2, z = −20; k = 9) (Fig. 1). In accordance with our hypothesis, amygdala activity in response to negative faces predicted negative evaluation shifts (valence-congruent priming) elicited by negative faces in the affective priming task. The whole-brain analysis yielded no significant activation in areas without an explicit a priori hypothesis.
Coronal views ranging from y = −4 to y = 0 depicting amygdala activity in response to masked negative (angry and sad) faces significantly correlated with bias scores for negative faces in the masked affective priming task (automatic negative judgmental bias). Voxel threshold was set at p < 0.05, with false discovery rate correction for bilateral amygdala volume.
Correlation analyses for each emotion separately yielded significant correlations of amygdala activity in response to angry and sad faces with bias scores for corresponding emotion. As hypothesized, stronger amygdala responses to masked angry and sad faces were associated with more negative bias scores elicited by corresponding negative prime faces in the affective priming task. However, this association seems to be confined to negative emotions, since amygdala responses to masked happy faces were not significantly correlated with evaluative shifts elicited by happy faces in the affective priming task. The predictive effect of amygdala activity on automatic negative judgmental bias was unaffected by trait anxiety or performance in the detection task (Table 2). Detection task performance was not correlated with bias scores or amygdala activity.
Pearson’s product-moment correlations between bias scores in the masked affective priming task and amygdala response (left and right) to corresponding facial emotions (n = 28)*
Role of medication
To assess the role of antidepressant medication, all medications were coded in terms of treatment duration and dosage into medication levels from 1 to 4 for each testing session separately, following the suggestions of Sackeim.39 Patients were grouped into either a low-dose group (medication level 1–2; n = 12) or a high-dose group (medication level 3–4; n = 16).40 The low-dose group showed significantly higher BDI scores than the high-dose group (t26 = 2.18, p = 0.039). However, no effect of medication on other clinical characteristics, bias scores or amygdala responses reached a trend level of significance (all p > 0.10).
Correlation analysis of amygdala responses, negative bias and clinical characteristics
Five variables characterizing illness history were assessed (duration of illness, number of episodes, total hospitalization time, time since first inpatient treatment and time since first outpatient treatment; see Table 1). All variables were entered into a factor analysis using principle component extraction. A clear single component solution emerged that extracted 60.6% variance and obtained high loadings (> 0.7) from all clinical variables. For each subject, component values were calculated according to the Anderson-Rubin method to get a “severity index.” Thus patients with a high severity index had a longer history of depression and more episodes and spent more cumulative time in hospital.
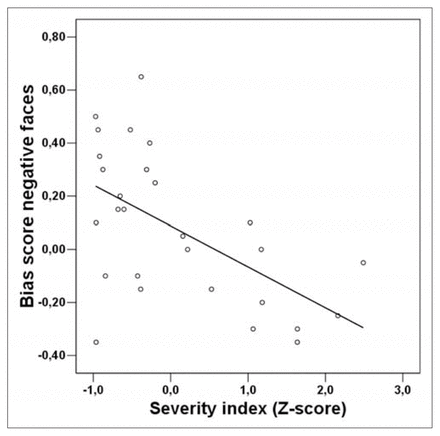
Correlations between left and right amygdala activity in response to negative faces and the severity index did not reach significance (r = 0.23, p = 0.24; r = 0.32, p = 0.10). However, a highly significant correlation emerged between judgmental bias elicited by negative faces and severity index (r = −0.57, p = 0.001) (Fig. 2). Patients with strong negative judgmental bias had higher scores on the severity index, indicating a longer and more severe course of disease. The correlation remained stable even when age, medication level and HAMD score were controlled (rp = −0.56, p = 0.003). Current depressive symptoms (according to HAMD and BDI scores) and trait anxiety (assessed by the STAI-trait) did not correlate with bias scores or amygdala activity.
Scatter plot depicting the correlation between bias score in the affective priming task for negative faces and the severity index (r = −0.57, p = 0.001). Negative values of bias scores indicate negative judgmental bias. The severity index represents component scores extracted from clinical variables and indicates duration and severity of illness. Positive severity index scores reflect a less benign course of disease.
Discussion
The present study replicated and extended previous findings concerning the association between amygdala reactivity to masked negative facial emotions and automatic negative judgmental bias.22 Activation of the amygdala in response to negative social cues seemed to evoke negative judgmental biases even if the patients were unaware of the emotional stimuli. Patients displaying stronger amygdala responses for covert negative facial affect showed significantly higher negative biases on an automatic level of emotion processing. Again, no association was found between priming based on happy faces and amygdala activity. It seems as if positive stimuli are processed differently in the amygdala than negative stimuli. Corresponding with the present data, Suslow and colleagues41 reported that amygdala activity in response to masked threat-related faces (angry and fearful), but not to happy expressions, could predict conscious detection of the stimuli.
The association of amygdala reactivity and negative bias occurred independently of the patients’ anxiety disposition, detection task performance or current medication level. In addition, 33% variance of the clinical course characteristics was explained by negative judgmental bias in the affective priming task. Thus patients displaying unconscious evaluative shifts elicited by masked negative faces seem to have a less benign course of disease, as indicated by their clinical history (e.g., more previous episodes and longer lifetime hospitalization). The correlation between amygdala activity in response to negative faces and the severity index failed to reach statistical significance. It appears that the psychological variable automatic negative judgmental bias is more closely related to illness course than the neurobiological variable automatic amygdala responsivity to negative faces during active viewing (in a memory task). Even though both variables were substantially correlated, sensitivity for the biasing influence of negative facial expression in a context of interpersonal appraisal appears to be more relevant for the onset and persistence of depression than (involuntary) encoding processes related to negative faces. To clarify whether automatic amygdala responsivity to negative faces in an appraisal setting is as effective in the prediction of illness course in depression as negative judgmental bias, we continue our research with an affective priming task administered directly in the MRI scanner.
The present findings fit well with neurobiological models of affective disorders in which amygdala hyperactivity is being discussed.16 Amygdala hyperactivity, particularly in response to negative stimuli, is a frequent finding in depression7–11 and anxiety disorders.42 Consequently, our data might suggest that pathological amygdala hyperactivity could affect the onset and maintenance of emotional disorders by eliciting dysfunctional negative biases already at automatic stages of affective information processing. An automatic negative judgmental bias was previously shown to be a predictive factor for a weak therapy response.6 Thus negatively biased emotion processing might represent a determinant for a longer and more severe course of depression, with amygdala hyperactivity being the corresponding substrate.
Certain limitations should be acknowledged. The affective priming data were collected in a separate experiment outside the scanner, and no behavioural data were collected during the fMRI session. However, acceptable test–retest characteristics of automatic affect processing have been reported for the affective priming task,43 suggesting that masked affective priming reflects temporally stable automatic processing characteristics. Further, viewing has the advantage of being unconfounded by task demands10 and was successfully employed to investigate amygdala activity in several previous experiments.20,41,44
It might be considered problematic that the affective priming task did not produce significant bias scores in our sample. However, these data are in line with a previous study45 and our own data6 (priming compared with the neutral-face baseline). It should be noted that, in healthy subjects, a positive bias based on masked happy faces is a robust finding in the affective priming literature,6,46–48 which includes our previous sample of healthy subjects measured with the identical paradigm. A direct comparison between the healthy sample and the present patient sample indicated that the patients seemed to have “lost” this positive bias elicited by happy faces (indicated by a significantly lower bias score for positive faces in comparison with a healthy sample reported previously22) (t47 = 2.1, p = 0.038), although no differences in bias scores elicited by negative faces emerged (ts47 < 1).
Our result showing a reduced sensitivity to positive facial expression on an automatic processing level is consistent with a large body of evidence indicating a general hyporesponsivity to positive emotional stimuli in depression. Implicit and explicit attentional biases away from happy stimuli have been repeatedly demonstrated in persons suffering from depression.49–51 There is evidence from neurobiological studies that healthy persons allocate more resources to positive information, whereas those suffering from depression lack such a positive cognitive bias.52 In patients with depression, a negative correlation was recently found between depression severity and magnitude of neural response to happy facial expression within cortical face-processing areas.40 Insofar as persons suffering from depression appear to already manifest a reduced sensitivity to positive stimuli at a very early processing stage, it cannot be expected that training interventions exercising voluntary attention to positive stimuli will easily modify biases in depression. Central characteristics of automatic affect processing such as affective priming are effortlessness, low controllability and high efficiency.53
In sum, we conclude that automatic amygdala reactivity to negative facial emotions appears to be a determinant of automatic negative judgmental bias in depression patients that, in turn, is associated with duration and severity of illness. The present data bridge a gap between neurobiological abnormalities, dysfunctional emotion processing and clinical characteristics. Combining fMRI and experimental cognitive neuropsychology can help to clarify not only where dysfunctional neural activity occurs but also how it affects emotion processing and contributes to clinical symptoms. Further research should make use of longitudinal designs, preferably with unmedicated patients, to clarify the predictive effect of negatively biased emotion processing as measured with fMRI and the affective priming task on the course of major depression.
Acknowledgements
This study was partly supported by an IMF grant (AR 510403) of the Medical Faculty of the University of Münster.
Footnotes
Medical subject headings: depressive disorder, major; magnetic resonance imaging, functional; amygdala; emotion.
Competing interests: None declared.
Contributors: Drs. Dannlowski, Ohrmann, Kugel and Suslow designed the study. Dr. Dannlowski, Mr. Bauer, and Drs. Kugel, Kersting, Baune and Suslow acquired the data, which Dr. Dannlowski, Mr. Bauer, and Drs. Kugel, Arolt, Heindel and Suslow analyzed. Drs. Dannlowski and Suslow wrote the article. Dr. Ohrmann, Mr. Bauer, and Drs. Kugel, Arolt, Heindel, Kersting, Baune and Suslow revised it. All authors gave final approval for the article to be published.
- Received December 18, 2006.
- Revision received April 4, 2007.
- Accepted May 6, 2007.