Abstract
Background: In recent years, a role for the immune system in the pathogenesis of psychiatric diseases has gained increased attention. Although bipolar disorder appears to be associated with altered serum cytokine levels, a putative immunological contribution to its patho-physiology remains to be established. Hitherto, no direct analyses of cerebrospinal fluid (CSF) cytokines in patients with bipolar disorder have been performed.
Methods: We analyzed CSF cytokine concentrations in euthymic patients with diagnosed bipolar disorder type I (n = 15) or type II (n = 15) and healthy volunteers (n = 30) using an immunoassay-based protein array multiplex system.
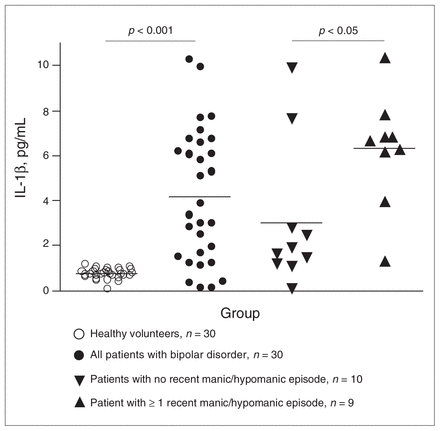
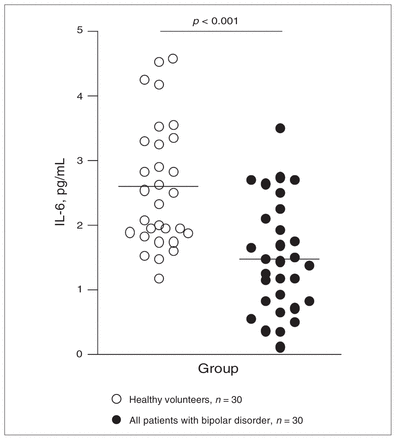
Results: The mean interleukin (IL)-1β level (4.2 pg/mL, standard error of the mean [SEM] 0.5) was higher and the IL-6 level (1.5 pg/mL, SEM 0.2) was lower in euthymic bipolar patients than in healthy volunteers (0.8 pg/mL, SEM 0.04, and 2.6 pg/mL, SEM 0.2, respectively). Patients with 1 or more manic/hypomanic episodes during the last year showed significantly higher levels of IL-1β (6.2 pg/mL, SEM 0.8; n = 9) than patients without a recent manic/hypomanic episode (3.1 pg/mL, SEM 1.0; n = 10).
Limitations: All patients were in an euthymic state at the time of sampling. Owing to the large variety of drugs prescribed to patients in the present study, influence of medication on the cytokine profile cannot be ruled out.
Conclusion: Our findings show an altered brain cytokine profile associated with the manifestation of recent manic/hypomanic episodes in patients with bipolar disorder. Although the causality remains to be established, these findings may suggest a pathophysiological role for IL-1β in bipolar disorder.
Introduction
Bipolar disorder is characterized by recurrent episodes of manic or depressive mood interspaced with periods of euthymia. The underlying pathophysiology of the disease is poorly understood, although manic symptoms have been attributed to an increased dopaminergic drive.1
In recent years, a role of the immune system in the pathogenesis of psychiatric diseases has gained increased attention. In this regard, many investigators have focused on cytokines, proteins that directly initiate and control immunological responses. We recently demonstrated a selective activation of brain interleukin (IL)-1β in schizophrenia,2 a disease that appears similar to bipolar disorder with regard to symptomatology, treatment and risk genes.3,4 Interestingly, a polymorphism in the promoter region of the IL1B gene has been suggested to comprise a shared genetic susceptibility for bipolar disorder and schizophrenia.5 Indeed, bipolar disorder appears to be linked to inflammation, as indicated by genetic polymorphisms, gene expression and cytokine activation,6 although direct evidence for a pathophysiological involvement of the immune system of the brain is still sparse. This may be related to the fact that evaluation of cytokine levels in patients with bipolar disorder has been restricted to serum analyses. The bidirectional communication between the immune system of the brain and that of peripheral organs is complex,7 and serum cytokines do not predict brain cytokine activation in healthy volunteers.8 Hence, the immune system of the brain, including local cytokine release from microglia and astrocytes, may be independent of the immune system in peripheral organs. The aim of the present study was to investigate an immune activation in the brains of patients with bipolar disorder by analyzing well-characterized cytokines in the cerebrospinal fluid (CSF).
Methods
Participants
We recruited patients meeting the DSM-IV criteria for bipolar disorder type I or II from the St. Göran bipolar project, run at a bipolar outpatient unit at the Northern Stockholm Psychiatric Clinic, Stockholm, Sweden. The work-up and diagnostic assessments have been described in detail previously.9 Briefly, the clinical diagnosis of bipolar disorder was established according to the structured interview the Affective Disorder Evaluation (ADE), which has previously been employed in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) project.10 To minimize inter-rater variability, a best-estimate diagnosis was achieved by presenting the collected information at a diagnostic case conference where a consensus panel of experienced board-certified psychiatrists specialized in bipolar disorder made the final diagnostic decision.
Cerebrospinal fluid sampling was performed when the participants were in a stable euthymic mood, as judged by a physician. For ethical reasons, patients continued to take their prescribed medications.
We recruited healthy volunteers among medical students, hospital staff members and their relatives at the Linköping University Hospital, Sweden. The volunteers had to be medication-free for at least 1 month and free from any form of substance abuse. Smoking was allowed. The healthy volunteers underwent laboratory tests (electrolytes, blood, kidney, liver and thyroid), a physical examination and a semistructured interview using the Structured Clinical Interview for DSM-IV (SCID) Axis I11 and Axis II12 Disorders questionnaires to determine eligibility for the study. Cytokine levels of healthy volunteers were analyzed and published in a previous study.2
After complete description of the study to the participants, we obtained written informed consent. The work described in the present study was carried out in accordance with the code of ethics of the world medical association (Declaration of Helsinki) for experiments including humans. The ethical committees of the Linköping University Hospital and the Karolinska Institutet, Sweden, approved our study protocol.
Lumbar puncture
Lumbar puncture was performed on all participants between 8 am and 11 am after a night of fasting and bedrest. A disposable needle (BD Whitacre Needle, 0.7 × 90 mm) was inserted at the L4–L5 level. A volume of 12 mL of CSF was collected, inverted to avoid gradient effects, divided into aliquots and stored at –70°C until analyzed.
Cytokine analysis
We used a sandwich immunoassay-based protein array multiplex system (Invitrogen AB) with a guaranteed lowest detection limit of 1 pg/mL for each cytokine to quantify IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, granulocyte-macrophage colony-stimulating factor (GM)-CSF, interferon (IFN)-γ and tumour necrosis factor (TNF)–α. The samples were incubated with beads coated with the specific antibodies. Thereafter, we conducted incubations with biotin-conjugated antibodies and streptavidin-phycoerythrin. Standard curves (Biosource International) ranging from 0.38 pg/mL to 1025 pg/mL of the respective cytokine were used for quantification. We used a Luminex reader (Luminex Corporation) to simultaneously quantify the concentrations of the cytokines.
Statistical analysis
We used GraphPad Prism 4.0c (GraphPad Software, Inc.) in all statistical analyses. Cytokine values are given as means and standard errors of the mean (SEM). We obtained correlations between variables using Spearman rank correlation. Group differences were established by 2-tailed Mann–Whitney U test. Significance was assumed for all values of p < 0.05.
Results
We enrolled 30 male patients (mean age 43.2 yr, standard deviation [SD] 13.5) in the study; 15 met the DSM-IV criteria for bipolar disorder type I and 15 for type II. Patients were taking the following medications: lithium (57%), lamotrigine (27%), quetiapine (20%), valproate (20%), mirtazapine (13%) and propiomazine (13%). Two patients were untreated at the time of sampling. Five patients were smokers. Detailed information about previous mood episodes was available for 19 of the 30 patients. Of these, we considered 3 patients to be outliers with regard to their total lifetime mood episodes; 70, 74 and 80 episodes, respectively, compared with the remaining 16 patients with a history of 1–25 mood episodes. For 1 patient, information on total number of lifetime depressive episodes was lacking. We included 30 healthy male volunteers (mean age 25.4, SD 7.2 yr) in the study.
Three of the cytokines analyzed, IL-1β, IL-6 and IL-8, were consistently detectable in all patients and healthy volunteers. The remaining cytokines analyzed were either undetectable or close to the detection limit of the assay.
As shown in Figure 1, IL-1β was markedly increased in patients (4.2 pg/mL, SEM 0.5) compared with healthy volunteers (0.8 pg/mL, SEM 0.04, U = 118, p < 0.001). In contrast, mean levels of IL-6 were lower in patients (1.5 pg/mL, SEM 0.2) than in healthy volunteers (2.6 pg/mL, SEM 0.2, U = 171.5, p < 0.001; Fig. 2). The patients’ IL-8 levels (75 pg/mL, SEM 10) did not differ from those in healthy volunteers (90 pg/mL, SEM 3).
Interleukin (IL)-1β in cerebrospinal fluid (CSF) of healthy volunteers and patients with bipolar disorder, with or without recent manic/hypomanic episodes (during the year preceding CSF sampling). Each point represents the concentration of IL-1β in a single CSF sample. Horizontal lines show mean values for each group. The p values were determined by Mann–Whitney U test.
Interleukin (IL)-6 in cerebrospinal fluid (CSF) of healthy volunteers and patients with bipolar disorder. Each point represents the concentration of IL-6 in a single CSF sample. Horizontal lines show mean values for each group. The p values were determined by Mann–Whitney U test.
Most patients were also analyzed with regard to their mood episode history. Patients with recent manic or hypomanic episodes (≥ 1 during the year preceding CSF sampling) had higher levels of IL-1β (6.2 pg/mL, SEM 0.8, n = 9) than those without recent manic/hypomanic episodes (3.1 pg/mL, SEM 1.0, n = 10, U = 20, p = 0.044). These patients did not differ with respect to diagnostic subtype (bipolar disorder type I or II), age or the lifetime number of manic/hypomanic episodes. Recent episodes of depression (≥ 1 in the year preceding CSF sampling) did not correlate to cytokine levels (data not shown). However, the total number of lifetime episodes of depression was found to negatively correlate with the CSF levels of IL-1β (Spearman correlation = –0.52, n = 15, p = 0.048). With regard to IL-6, no correlations with recent or lifetime number of mood episodes were observed.
No associations were found between IL-1β, IL-6 and IL-8 levels and diagnosis (bipolar disorder type I or II), ongoing medication or smoking (data not shown). Furthermore, these cytokine levels did not correlate with age in patients or in healthy volunteers.
Discussion
The present results show an increased concentration of IL-1β in lumbar CSF of euthymic patients with bipolar disorder, likely reflecting an immune activation in the central nervous system (CNS). The increased concentration of CSF IL-1β is in excellent agreement with a recent study showing increased mRNA levels of IL-1β in the postmortem brains of patients with bipolar disorder.13 Given the similarities between schizophrenia and bipolar disorder, this finding is also in line with a recent study from our group showing increased concentration of this cytokine in the CSF of patients with a first-episode of schizophrenia.2
The presently observed aberrant CSF cytokine profile in euthymic patients with bipolar disorder (i.e., a selective activation of IL-1β concomitant with a reduction of IL-6) clearly differs from previous findings where cytokines of serum have been analyzed.14–16 Although this discrepancy may relate to fluctuations of cytokines along with various states of the disease, the cytokine profile of the brain may also clearly differ from that of the periphery.8,17,18
Supporting a central origin of the presently observed increased IL-1β concentration, a recent postmortem study demonstrated higher protein and mRNA levels of IL-1β in patients with bipolar disorder.13 However, since peripheral cytokines may access the brain through a damaged blood–brain barrier or through volume diffusion where the barrier is absent, such as in paraventricular organs, a tentative contribution of peripheral cytokines to the brain in bipolar disorder should not be disregarded.
The observed change in CSF cytokine profile is not to be expected during a CNS infection. However, proinflammatory cytokines released from microglia and astrocytes in the brain may, in addition to their classical role in immune responses, be involved in the tonic control of numerous brain processes affecting behaviour. Thus, IL-1β has been attributed a fundamental role in sickness behaviour.7 The action of IL-1β on neuronal circuits appears complex and diverse, including direct as well as indirect effects on excitatory and inhibitory neurotransmission.19,20 It was recently shown that kynurenic acid (KYNA) is elevated in the CSF of patients with bipolar disorder.21 Numerous studies have shown that this compound, being an end product of tryptophan degradation along the kynurenine pathway, may serve as a marker of immunoactivation.7 Thus, several cytokines are able to induce or repress critical enzymes of the kynurenine pathway like indoleamine 2,3-dioxygenase (IDO) and kynurenine 3-monooxygenase (KMO),22,23 leading to increased KYNA production. Kynurenic acid is an antagonist at the glycine site of the N-methyl-d-aspartate receptor24,25 as well as at the cholinergic α7* nicotinic receptor,26 and hence provides a potential link between the immune system and glutamatergic/cholinergic neurotransmission. The elevation of KYNA in CSF from patients with bipolar disorder may therefore be causally related to the presently shown activation of IL-1β.
Present results also demonstrate an association between IL-1β levels and the core symptoms of bipolar disorder. Thus, IL-1β is significantly higher in patients with recent episodes of mania/hypomania, whereas the lifetime number of depressive episodes is negatively correlated to IL-1β CSF levels. These findings may be indicative of a fluctuation of this cytokine along the various states of bipolar disorders. Thus, the relation between brain cytokine levels and mood state in bipolar disorder deserves further investigation.
Limitations
Most of the patients received pharmacological treatment at the time of CSF collection. Available studies show different effects of psychotropic drugs on cytokine levels. For example, valproate may elevate plasma levels of IL-1β and IL-6,27 whereas antidepressants and lithium generally have negative immunoregulatory effects, as reviewed by Maes.28 Clearly, a putative influence of medication on CNS cytokine expression may be a confounding factor in the interpretation of the present results.
Conclusion
The present study demonstrates an aberrant profile of immunologically active molecules (i.e., a selective increase in IL-1β concomitant with a reduction of IL-6) in the CSF of patients with bipolar disorder compared with healthy volunteers. In addition, we observed a direct association between cytokine levels and mood episodes. Although additional studies are needed to address causality, our findings clearly show that bipolar disorder is associated with a change in the CNS cytokine profile.
Acknowledgements
We gratefully acknowledge patients and healthy volunteers for their participation in the study and express our gratitude toward health professionals who facilitated our work. In particular, we thank study coordinator Mrs. Martina Wennberg, and research nurses Mrs. Agneta Carswärd-Kjellin and Mrs. Stina Stadler.
Footnotes
↵* These authors contributed equally to this work.
Conflicts of interest: None declared.
Contributors: Drs. Söderlund, Landén, Erhardt and Engberg designed the study. Drs. Samuelsson, Walther-Jallow, Johansson, Landén and Engberg acquired the data, which Drs. Söderlund, Walther-Jallow, Landén and Engberg and Ms. Olsson analyzed. Drs. Söderlund, Landén, Erhardt and Engberg and Ms. Olsson wrote the article, which Drs. Söderlund, Samuelsson, Walther-Jallow, Johansson, Landén and Ms. Olsson critically reviewed. All author approved publication of the article.
Funding: Financial support for this study was provided through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet, the Östergötland County Council, the Swedish Brain Foundation, Svenska Läkaresällskapet, Karolinska Institutet and the Swedish Research Council (Dr. Erhardt, No. 2009–4046; Dr. Engberg, 2008–3822, 2009–3068; Dr. Landén, K2008–62x-14647–06–3.
- Received May 18, 2010.
- Revision received August 3, 2010.
- Accepted August 4, 2010.