Abstract
Background: An attentional bias to health-threat stimuli is assumed to represent the primary pathogenetic factor for the development and maintenance of pathological health anxiety (PHA; formerly termed “hypochondriasis”). However, little is known about the neural basis of this attentional bias in individuals with PHA.
Methods: A group of patients with PHA, a group of depressed patients and a healthy control group completed an emotional Stroop task with health-threat (body symptom and illness) words and neutral control words while undergoing functional MRI.
Results: We included 33 patients with PHA, 28 depressed patients and 31 controls in our analyses. As reflected in reaction times, patients with PHA showed a significantly stronger attentional bias to health-threat words than both control groups. In addition, patients with PHA showed increased amygdala and rostral anterior cingulate cortex activation for body symptom, but not for illness words. Moreover, only in patients with PHA amygdala activation in response to symptom words was positively associated with higher arousal and more negative valence ratings of the body symptom word material.
Limitations: A control group of patients with an anxiety disorder but without PHA would have helped to define the specificity of the results for PHA.
Conclusion: The attentional bias observed in patients with PHA is associated with hyperactivation in response to body symptom words in brain regions that are crucial for an arousal-related fear response (e.g., the amygdala) and for resolving emotional interference (e.g., the rostral anterior cingulate cortex). The findings have important implications for the nosological classification of PHA and suggest the application of innovative exposure-based interventions for the treatment of PHA.
Introduction
Pathological health anxiety (PHA), formerly termed “hypochondriasis,” represents a complex, costly and therapeutically challenging clinical condition.1 Traditionally, PHA was considered a somatoform disorder in DSM-IV,2 and it is now classified within somatic symptom and related disorders in DSM-5.3 Despite this classification, recent theoretical considerations and empirical findings4,5 have suggested that PHA might be better conceptualized as an anxiety disorder or an obsessive–compulsive spectrum disorder (as planned for ICD-11). A better understanding of the crucial cognitive, emotional and neural mechanisms involved in the pathogenesis of PHA appears to be of paramount importance, not only for solving the classification issue, but also to foster the development of more effective treatment options.
Phenomenologically, PHA is characterized by the preoccupation of having a serious disease.6 Despite no evidence for a serious organic pathology after appropriate medical evaluation, patients with PHA remain convinced of having a severe illness and show permanent alertness and hypervigilance for potentially internal and external health-threat information.7–9 On a behavioural level, this preoccupation manifests in efforts gathering illness-related information, engaging in body-checking behaviour and seeking medical reassurance, or in avoiding medical appointments owing to the conviction of being unable to handle the explicit diagnosis of the assumed life-threatening illness.
Cognitive behavioural models of PHA emphasize 3 pivotal abnormalities (“biases”) in the processing of health-relevant information:8–10 increased attention allocation to bodily sensations, catastrophic misinterpretation of minor bodily sensations as potential signs of a severe illness, and limited ability to distract from illness-signalling information and to inhibit catastrophic interpretations. From a neurobiological perspective, increased attention allocation and catastrophic interpretation of bodily sensations as threatening is associated with stimulus-induced affective arousal mediated by enhanced amygdala activation,11 whereas the disability to distract from relevant cues has been found to be related to reduced pre-frontal inhibitory mechanisms.12
An experimental paradigm in which both an increased attention for and reduced inhibition of emotionally negative and individually salient contents are assumed to affect overt behaviour is the emotional Stroop task (EST).13 The EST has been shown to be useful for eliciting emotional responses14 and for affecting controlled cognitive processing.15 In 2 elegant EST fMRI studies, Egner and colleagues16 and Etkin and colleagues17 showed that the amygdala is strongly associated with detecting the emotional salience of stimuli, whereas the rostral anterior cingulate cortex (rACC) plays a pivotal role in emotional conflict/interference reduction. The EST is one of the earliest and most frequently used measure of attentional bias across psychopathologies.13 Whereas earlier theories about cognitive processes in individuals with depression and anxiety have postulated that biased attention allocation (as assessed with the EST) would be specific for those with anxiety disorders compared with depressive disorders,18 more recent meta-analytic reviews on the attentional bias as measured with the EST have revealed significant small- to medium-sized emotional interference effects in individuals with subclinical and clinically relevant anxiety19 and depression.20,21 Existing evidence on the question of a possible causal link between attentional bias and anxiety points to a rather bidirectional association in which biased attention allocation is able to increase symptoms of anxiety and vice versa.22
Although previous studies reliably demonstrated stronger EST effects in response to health-threat words in individuals with subclinical health anxiety23 as well as in patients with PHA,24–26 to our knowledge, there is only 1 fMRI study that focussed on the neural correlates of this effect in patients with PHA. van den Heuvel and colleagues25 used an EST with panic disorder–specific word material and compared patients with panic disorder to patients with PHA. The authors showed that patients with PHA had an attentional bias to panic-related words, as reflected in response slowing on the behavioural level and neural hyperactivation in a broad frontostriatal network.25 However, in contrast to patients with panic disorder, these hyperactivations did not extend to the amygdala and cingulate regions.
Witthöft and colleagues23 applied an EST with specific word stimuli for health anxiety (i.e., illness words indicating a severe disease, such as tumour and stroke, and symptom words indicating bodily complaints, such as cough and sweating) during fMRI in participants with varying subclinical health anxiety. Those with higher levels of health anxiety showed marked emotional interference, especially in response to symptom words. This behavioural EST effect was related to aberrant activation in the left rACC,23 providing evidence for dysfunctions in emotional interference resolution, particularly to symptom-related words, in individuals with subclinical health anxiety. It remains unclear, however, whether these findings derived in a subclinical sample can be generalized to patients with PHA.
To investigate aberrations in attention allocation and inhibition of health-threat contents in patients with PHA, we administered an EST with aversive health-threat words (body symptom and illness words) among patients with PHA during fMRI. Two control groups were included: patients with depression and healthy control participants. Patients with depression are known to be responsive to the EST and to show altered emotional processing and aberrant rACC and amygdala functioning.27–29 Hence, patients with depression are a suitable comparison group in which to investigate the specificity of alterations in these brain regions in patieints with PHA. We hypothesized that patients with PHA would show a stronger emotional reaction when confronted with health-threat stimuli than both control groups, as reflected in increased amygdala activation. Based on previous findings related to reduced capabilities of emotional conflict resolution, we expected patients with PHA to show higher rACC activation than participants in either of the control groups.
Methods
Participants
We included 92 participants from a larger study on PHA: 33 patients with PHA, 31 healthy controls and 28 depressed controls (see26,30,31 for details). Participants were matched for age, sex and education. To be included in the present study, participants had to be right-handed and have normal or corrected-to-normal vision. Prior to enrolment in the study, participants were informed about study procedures and purpose and gave written informed consent. The Medical Ethics Committee of the Medical Faculty Mannheim at the University of Heidelberg, Germany, approved the study, which was conducted in concordance with the declaration of Helsinki. Prior to study inclusion, participants completed a set of screening questionnaires related to health anxiety and depression comprising the Short Health Anxiety Inventory,31 the Whiteley Index32 and the Patient Health Questionnaire depression (PHQ-9) and somatic (PHQ-15) symptom scales.33
In light of ongoing criticisms of the DSM-IV diagnosis of hypochondriasis and because DSM-5 had not yet been published when the study was conducted, the diagnosis of PHA was established using an interview based on the criteria proposed by Fink and colleagues34 (see Appendix 1, available at jpn.ca). In addition, the SCID interview35 was used to assess DSM-IV hypochondriasis and possible comorbidities in patients with PHA, to validate major depression or dysthymia in the depression group and to exclude mental disorders in the healthy control group.
Participants were included in the study only if they had no history of neurologic disease and met MRI inclusion criteria. Exclusion criteria were diagnoses of substance use disorder or schizophrenia. Additional exclusion criteria for the depression and healthy control groups were severe symptoms of health anxiety, comorbid panic disorder, obsessive– compulsive disorder, generalized anxiety disorder, or any somatoform disorder. In addition, affective disorders were an exclusion criterion for the healthy control group.
The complete study protocol involved several appointments, including ratings of emotional valence and arousal of the word stimuli with the self-assessment mannequin (SAM)36 to evaluate the suitability of the word material.
Experimental design
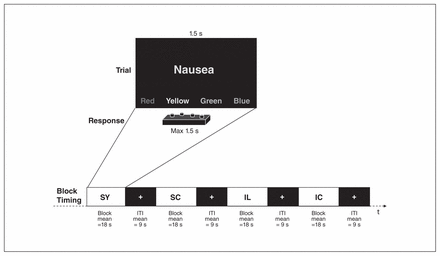
We administered an EST23 with words from 4 categories: symptom words referring to common bodily complaints or sensations (e.g., dizziness, headache, nausea), illness-related words referring to triggers and consequences of real physical disease processes (e.g., tumour, cancer, suffocation) and neutral stimulus categories of kitchen words and furniture items matched by word length and frequency in German language to the symptom and illness words, respectively. Words were presented in different colours (red, green, yellow and blue), and participants were asked to indicate the colour of the word by pressing a button as fast as possible while ignoring the word content. Figure 1 shows the experimental paradigm. Total experimental time was about 7 min (for details see Appendix 1).
The Emotional Stroop Task (EST). IC = illness control words; IL = illness words; ITI = intertrial interval; SC = symptom control words, SY = symptom words.
Prior to the experiment, participants practised the task in the scanner in a short training session using rows of letters.
Functional MRI data acquisition
Data were obtained using a 3 T Siemens Tim TRIO whole-body magnetic resonance tomograph (Siemens Medical Systems). Prior to functional imaging a T1-weighted anatomic scan was acquired from all participants (162 slices, 1 × 1 × 1 mm voxel size). For collection of the functional images we used a T2*-weighted gradient echo planar imaging (EPI) sequence with the following parameters: repetition time (TR) 2000 ms, acquisition time (TA) 100 ms, echo time (TE) 50 ms, flip angle 90°, field of view 224 mm, 64 × 64 matrix. Each volume contained 28 slices, collected in a descending order with a slice-thickness of 3 mm with a 1 mm gap (resulting voxel size 4 × 3 × 3 mm3).
Statistical analysis
The description of the analysis of the behavioural data can be found in the Appendix. We analyzed fMRI data using SPM8 software (www.fil.ion.ucl.ac.uk/spm/software/spm8/). Data preprocessing included slice time correction to the temporal middle slice, realignment to the average EPI, spatial normalization with a 3 × 3 × 3 mm voxel size to the Montreal Neurological Institute (MNI) template and spatial smoothing (8 mm full-width at half-maximum kernel). For the first level fixed-effects analyses, a general linear model was set up with the onsets of the condition as regressors of interest and the 6 movement parameters derived from realignment as regressors of no interest. Data were analyzed in a block design fashion, modelling the duration of each block, folded with a boxcar function.
Analyses on the group level were accomplished by subjecting the contrasts from the first level into the second level random-effects analyses, applying the most ecological and to the best of our knowledge most statistically sound statistical model currently available in SPM8. First, a flexible factorial design was applied that allowed the investigation of the main effect of health threat as well as the group × health-threat interaction. Second, a full factorial design was set up to analyze the main effect of group. Third, analyses of the group × health-threat interaction were achieved separately for the 2 types of stimuli (symptom words > control words; illness words > control words) using 1-way analyses of variance (ANOVA). We performed post hoc comparisons (PHA v. depression control group; PHA v. healthy control group; healthy control v. depression control group) using 2-sample t tests to analyze differences between groups for symptom words (> control words) and for illness words (> control words). We used 2 regions of interest (ROIs): the amygdala and rACC. The amygdala mask was derived from the Wake Forest University (WFU) PickAtlas. The rACC mask was adapted from the study by Egner and colleagues16 and proven to be suitable for the current EST task.23 We set the significance threshold for ROI analyses to p < 0.05, with a threshold of p < 0.05, small-volume corrected (SVC; i.e., peak voxel significance survives family-wise error [FWE] correction). Masks were not clustered together into a single mask, and no correction for the number of masks was applied. We set the significance threshold for whole brain analyses to p < 0.05, FWE-corrected. Cluster size threshold was set to k = 5 adjacent voxels. In addition, first eigenvariates of the left and right rACC and the left and right amygdala were extracted from the symptom words contrast (without applying a significance p threshold to assure the same number of voxels within each mask for all participants) to analyze the association between the processing of the body symptom words during the EST and the explicit rating of the body symptom words with the SAM. We used the eigenvariate, because it summarizes the responses but does not assume homogeneous signal within an ROI.37 These analyses, as well as the analysis of the behavioural data were accomplished using SPSS software version 20.
Results
Sample characteristics
Sample characteristics as well as screening questionnaire results and diagnoses are presented in Table 1. Ten participants in the PHA group were taking antidepressants, 1 was taking antipsychotics, and 1 took a tranquilizer when needed. In the depression control group, 11 participants were taking antidepressants and 1 took a tranquilizer when needed. No participants in the healthy control group took psychotropic medication.
Demographic and clinical characteristics of study participants
Behavioural data and SAM ratings
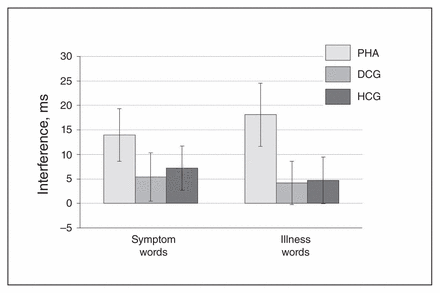
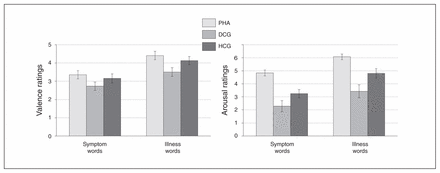
Patients with PHA showed a significantly stronger emotional interference (symptom and illness words v. control words) in the EST task and significantly higher arousal ratings for the health threat–related word material in comparison to the control groups (Fig. 2 and Fig. 3, Appendix 1).
Emotional interference in the Emotional Stroop Task (EST). Displayed are reaction time differences (symptom > symptom control words on the left; illness > illness control words on the right), reported as means and standard errors. DCG = depression control group; HCG = healthy control group; PHA = pathological health anxiety group.
Self-assessment manikin ratings of health-threat words. Valence ratings appear on the left, and arousal ratings appear on the right. Displayed are rating differences (symptom > symptom control words; illness > illness control words on the right), reported as means and standard errors. DCG = depression control group; HCG = healthy control group; PHA = pathological health anxiety group.
Functional MRI data
For each of the following analyses, a whole brain comparison as well as ROI analyses were conducted. Only the significant and marginally significant results are reported.
The analysis of health-threat effects across all participants (symptom and illness words > control words) revealed significant activation in the temporal gyrus and inferior prefrontal gyrus (Table 2 and Fig. 4).
Main effect of health threat (symptom and illness words > symptom and illness control words; p < 0.05, family-wise error–corrected).
Functional brain imaging results for the main effect of health-threat words, group and the group × health-threat words interaction (p < 0.05, family-wise error–corrected)
Comparison of the 2 types of threat words (v. control words) across all participants revealed significantly stronger activation in the primary visual cortex bilaterally for symptom words than for illness words (left MNI coordinates: x, y, z = −9, −85, 4, t = 10.01, p < 0.001; right MNI coordinates: x, y, z = 12, −85, −2, t = 7.92, p < 0.001). In addition, a trend toward stronger right amygdala activation was revealed for illness words than for symptom words (MNI coordinates: x, y, z = 18, −4, −23, t = 2.39, pSVC = 0.10).
Comparison between groups, independent of word type, showed increased activation in the left angular gyrus in patients with PHA (Table 2). Furthermore, ROI analysis revealed stronger activation in the bilateral amygdala (left amygdala MNI coordinates: x, y, z = −27, −7, 23, t = 2.47, pSVC = 0.06; right amygdala MNI coordinates: x, y, z = 27, −7, −23, t = 2.76, pSVC = 0.029) and in the left rACC (MNI coordinates: x, y, z = −6, 47, 5, t = 3.58, pSVC = 0.005). The reverse contrast (healthy controls and depressed controls > patients with PHA) showed increased activation in the frontal gyri as well as the cerebellum, putamen and middle occipital gyrus (Table 2).
Analysis of a health threat (illness and symptom words > control words) × group interaction revealed increased activation in the bilateral amygdala (left amygdala MNI coordinates: x, y, z = −27, 2, −20, t = 2.83, pSVC = 0.020; right amygdala MNI coordinates: x, y, z = 21, −4, −26, t = 2.21, pSVC = 0.08) for the patients with PHA.
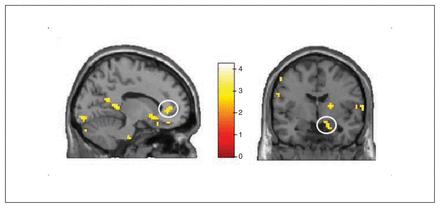
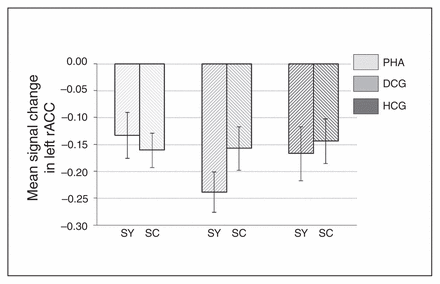
Whole brain analyses revealed no significant group differences for the symptom words (v. control words). However, ROI analyses revealed increased activation in the PHA group in comparison to the control groups in the bilateral rACC (left rACC MNI coordinates: x, y, z = −12, 41, 4, t = 2.86, pSVC = 0.032; right rACC MNI coordinates: x, y, z = 6, 44, −5, t = 2.71, pSVC = 0.045; Fig. 5 and Fig. 6) and in the bilateral amygdala (left amygdala MNI coordinates: x, y, z = −27, 2, −20, t = 2.58, pSVC = 0.042; right amygdala MNI coordinates: x, y, z = 18, −4, −20, t = 2.74, pSVC = 0.029; Fig. 5). Post hoc ROI comparisons for symptom words (v. control words) showed that patients with PHA had increased activation in the bilateral amygdala (left amygdala MNI coordinates: x, y, z = −21, −7, −23, t = 2.55, pSVC = 0.045; right amygdala MNI coordinates: x, y, z = 21, −7, −23, t = 3.12, pSVC = 0.012) and in the left rACC (MNI coordinates: x, y, z = −12, 44, 4, t = 2.70, pSVC = 0.048) in comparison to healthy controls. However, differences between patients with PHA and controls with depression did not reach significance.
Group × health threat interaction for the symptom words. Interactions in (left) the left rostral anterior cingulate cortex and (right) to the right amygdala. Threshold for display purposes p < 0.01, k = 10.
Signal change in the left rostral anterior cingulate cortex (rACC), separately for the 3 groups, as well as for symptom and symptom control words. Results are reported as means and standard errors. DCG = depression control group; HCG = healthy control group; PHA = pathological health anxiety group; SC = symptom control words; SY = symptom words.
For the illness words (v. control words), between-group whole brain comparisons revealed significantly increased activation in the right primary visual cortex in the PHA group (MNI coordinates: x, y, z = 12, −85, −2, t = 5.11, p = 0.016). The ROI analyses showed significantly increased right amygdala activation (MNI coordinates: x, y, z = 27, −7, −14, t = 2.49, pSVC = 0.049) in the PHA group compared with the control groups. In addition, right rACC activation was significantly higher in the control groups than in the PHA group (MNI coordinates: x, y, z = 15, 41, −5, t = 2.67, pSVC = 0.048). Post hoc comparisons for illness words (v. control words) revealed a trend toward hypoactivation in the right rACC (MNI coordinates: x, y, z = 12, −41, −5, t = 2.56, pSVC = 0.06) in patients with PHA compared with the healthy control group. In comparison to the depression control group, patients with PHA had increased activation in the right amygdala (MNI coordinates: x, y, z = 27, −7, −17, t = 3.71, pSVC = 0.003).
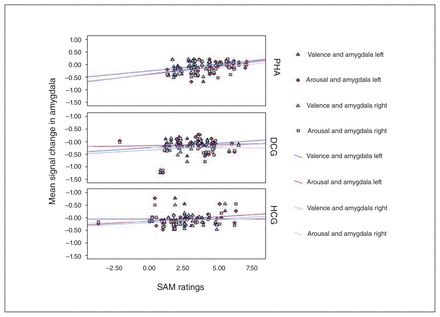
Moreover, significant correlations were revealed in patients with PHA between left and right amygdala activation in response to symptom words and explicit SAM arousal (left amygdala: r = 0.40, p = 0.020; right amygdala: r = 0.35, p = 0.048) and valence ratings (left amygdala: r = 0.33, p = 0.07; right amygdala: r = 0.43, p = 0.012). These associations were not significant in the control groups (all p > 0.14; Fig. 7). A significant association between the valance ratings and activation in left rACC (r = 0.53, p = 0.004) was revealed only in the depression control group. No other associations with rACC activation were significant.
Association between valence and arousal ratings for the symptom words and signal change in left and right amygdala in response to the symptom words during the emotional Stroop task. Correlations are displayed separately for the groups. DCG = depression control group; HCG = healthy control group; PHA = pathological health anxiety group; SAM = self-assessment mannequin.
Discussion
The present study aimed to clarify the neural bases of attentional bias to health threat, which is assumed to represent one of the pivotal processes in the etiology and maintenance of PHA. On the behavioural level, patients with PHA showed significantly stronger interference to health threatening words than participants in the healthy and depression control groups. In addition, patients with PHA rated the threatening stimuli as more arousing.
With regard to the neural correlates of this attentional bias, increased activity in the inferior prefrontal gyrus and temporal cortex during the confrontation with health-threat words was found across groups. Among activity increases in multiple broadly distributed brain areas, studies have frequently identified both the inferior prefrontal gyrus and the temporal cortex to be involved in the traditional Stroop task38–40 and the EST.25,41 However, these areas typically engaged in attentional control demands during (emotional) Stroop interference were found to be unaffected in patients with PHA.
Instead, patients with PHA showed increased activity in the left angular gyrus regardless of the type of word stimuli (heath threatening/neutral), whereas the control groups showed increased activation in brain areas associated with motor preparation. Remarkably, the only other fMRI study investigating emotional interference in patients with PHA also found increased activation in posterior areas, including the angular gyrus.25 Given the known involvement of the angular gyrus in semantic processing and in the default mode,42 it appears plausible that increased angular gyrus activation in patients with PHA is associated with more focused attention to the content of information in settings or situations where it is highly likely that the individual will encounter potentially health-threatening cues. This interpretation is also in line with that of a study demonstrating better recognition performance for health-threat words in an EST in patients with PHA.24 Moreover, the angular gyrus is part of the temporoparietal junction, which has been linked to salience processing,43–45 suggesting a higher salience of health-threat words for patients with PHA. This interpretation is in agreement with a recent study from our group that provided evidence for altered salience processing of body symptom words in individuals with subclinical health anxiety.46 In addition to the hyperactivation in the angular gyrus, patients with PHA showed increased activation in the amygdala and the left rACC. This activation pattern suggests that enhanced attention to the word stimuli is linked to elevated emotional arousal and impaired interference reduction during EST performance.16,17
More specifically, the observed aberrations in rACC and amygdala activation seem to be driven mainly by group differences during the processing of health-threat words, particularly the symptom words. In a previous study with subclinically health-anxious participants,23 health anxiety was also associated with reduced deactivation in response to symptom words in the left rACC. In the present study, patients with PHA showed this activation pattern in the bilateral rACC, and they showed increased bilateral amygdala activation. The amygdala and rACC form a structurally47 and functionally48 connected circuit, central for emotion processing. Within this circuit, the rACC has been shown to have a modulatory influence on the amygdala that seems to be affected, for instance, in individuals with depression27 and in carriers of risk genes for neuroticism.48
In addition, a positive correlation of bilateral amygdala activation in response to body symptom words during the EST and explicit valence and arousal ratings of these words was observed in patients with PHA. These correlations suggest that body symptom information apparently acquires specific salience linked to increased negative emotional arousal and biased attention in patients with PHA.23,49,50 However, it should be kept in mind that despite the medium effect size, these correlations would not be significant after correction for multiple comparisons. Moreover, these results give evidence for an exaggerated amygdala activity in response to body symptom stimuli as the neural basis of the fear response that causes behavioural neutralization reactions, such as body checking behaviour, to reduce the emotional reaction. Hence, cognitive behavioural therapies may focus more on emotional reactions to stimuli that might predict health threat. Possibly, expanding the usage of techniques from the treatment of anxiety disorders, such as exploring the assumed consequences of a devastating illness that a body symptom might signal combined with reattribution training, or the application of exposure-based techniques, seems to be warranted. Recent intervention studies that focused on these procedures reported promising effect sizes.51,52
The present findings are of interest for the ongoing nosological debate on how to classify PHA. Although DSM-5 imposed fundamental changes on the former concept of hypochondriasis (including a new label and the fragmentation of the condition into 2 distinct diagnoses; see Rief and Martin53 for details), the condition is still classified among the former section of somatoform disorders, now labelled “somatic symptom and related disorders.“ In contrast, several studies have shown that PHA shares vast commonalities with anxiety disorders,4 implying that PHA, as currently covered in the novel DSM-5 diagnoses of somatic symptom disorder and illness anxiety disorder, should rather be classified among the anxiety disorders. Our findings of amygdala hyperactivation in reaction to health-threat words support this assumption and point to a proximity of PHA to the anxiety disorders.
Limitations
Limiting our conclusions is that many of the patients with PHA had comorbidities, particularly anxiety (e.g., panic disorder, 33.3%) and mood disorders (e.g., major depression, 27.3%). Hence, to ensure the activation pattern that resembles the one of patients with anxiety disorders cannot be attributed to the comorbidities, it would be interesting for future studies to include only patients with PHA without any co-morbidities. In addition, future studies should investigate the replicability of our results with patients with illness anxiety disorder diagnosed according to DSM-5. While the clinical control group of patients with depression suggests that the stronger response to health-threat words is specific to patients with PHA, it is not clear whether this result is due to generally increased emotional interference in response to negative stimuli, or whether the effect is specific for health-threat words. Thus, a limitation of the present study is the lack of a category with negative, but not health-threat words. Further, a clinical control group of patients with anxiety disorders would have allowed a direct investigation of the specificity of the stronger response in patients with PHA in comparison to other anxiety disorders.
Moreover, the applied EST does not allow disentangling increased attention allocation toward health-threat words from a reduced capacity to disengage attention of these stimuli. Thus, we see stronger emotional interference (in terms of response times) in patients with PHA that can be linked to attentional processes, but we cannot determine whether this is based on a disturbance in a more bottom–up or top–down attentional process. The fMRI results with increased amygdala and rACC activation suggest that both processes are affected. This, however, might be due a deficit in one of the processes that interferes with the other. Hence, further studies that disentangle these processes are warranted.
In addition, it should be mentioned that the group differences in amygdala and rACC activation were rather small. There may be several reasons. One explanation is that the attention bias is due to alterations in additional brain regions (e.g., the angular gyrus that was found for the main effect of group) or that not only brain activation is affected, but also the connectivity between brain regions. Another explanation is based on the considerable overlap in the existing categorical diagnoses for depressive disorders, hypochondriasis and other anxiety disorders (e.g., generalized anxiety disorder) in terms of their phenomenology (e.g., high levels of negative repetitive thinking) as well as their supposed underlying pathophysiology (e.g., alterations in amygdala activation). Therefore, further studies might benefit from refined diagnostic categories or from adopting a different diagnostic approach, such as the one proposed by the National Institute of Mental Health Research Domain Criteria initiative that relies on a dimensional approach instead of diagnostic categories.54,55
Conclusion
The present study, for the first time to our knowledge, clarifies the neural correlates of biased information processing regarding health-threat stimuli in patients with PHA. The findings point to a crucial role of alterations in the amygdala and rACC in light of health-threat stimuli, linked to the attentional bias and self-reported arousal in patients with PHA. The results suggest a proximity of PHA to the realm of anxiety disorders and support a more consistent usage of specific interventions that have been proven to be efficacious in the treatments of anxiety disorders.
Acknowledgements
This study was supported by the Deutsche Forschungsgemeinschaft (DFG BA1597/5-1). The authors thank the colleagues who helped with study organization, clinical ratings and data acquisition.
Footnotes
Competing interests: None declared.
Contributors: J. Bailer, F. Rist, M. Witthöft and C. Diener designed the study. D. Mier, J. Bailer, J. Ofer, T. Kerstner and V. Zamoscik acquired the data, which D. Mier, J. Bailer, V. Zamoscik, F. Rist, M. Witthöft and C. Diener analyzed. D. Mier, J. Bailer, M. Witthöft and C. Diener wrote the article, which all authors reviewed and approved for publication.
- Received April 26, 2016.
- Revision received August 29, 2016.
- Accepted September 25, 2016.