Abstract
Background: Approximately 35% of people with depression do not respond to 2 courses of antidepressant medications of adequate dosage, and treatment-resistant depression (TRD) is still a major clinical concern with a great impact on patients, their families, society and the health system. The present meta-analysis evaluates antidepressant efficacy of unilateral and bilateral repetitive transcranial magnetic stimulation (rTMS) in patients with unipolar TRD.
Methods: We searched for randomized controlled trials that compared rTMS with sham treatment and were published by Apr. 3, 2017. The primary outcome was improvement in depression scores measured using the Hamilton Rating Scale for Depression. The secondary outcomes were remission and response rates. Two independent review authors screened the studies and extracted the data.
Results: Twenty-three studies met the inclusion criteria. Meta-analysis of the depression scores showed a weighted mean difference (WMD) of 3.36 (95% confidence interval [CI] 1.85–4.88) between unilateral rTMS and sham treatment. Stratified data showed that the effect was relatively higher when rTMS was used as an add-on to antidepressant medications (WMD 3.64, 95% CI 1.52–5.76) than when it was used as a stand-alone treatment (WMD 2.47, 95% CI 0.90–4.05). The WMD between bilateral rTMS and sham was 2.67 (95% CI 0.83–4.51), and all studies that contributed to this outcome used rTMS while participants were taking antidepressant medications. The pooled remission and response rates for unilateral rTMS versus sham treatment were 16.0% and 25.1% for rTMS and 5.7% and 11.0% for sham treatment, respectively. The pooled remission and response rates for bilateral rTMS versus sham treatment were 16.6% and 25.4% for rTMS and 2.0% and 6.8% for sham treatment, respectively.
Conclusion: This study suggests that rTMS has moderate antidepressant effects and appears to be promising in the short-term treatment of patients with unipolar TRD.
Introduction
Treatment-resistant depression (TRD) is a debilitating mental illness with substantial morbidity resulting in the loss of quality of life for millions of people around the world. Despite the use of multiple and adequately dosed antidepressant medications, some individuals do not benefit sufficiently from pharmacotherapy. Poor outcomes and a lack of response may cause clinicians to move toward a nonpharmacological treatment strategy. In this context, issues of effectiveness and safety become critically important. On the basis of data from randomized controlled trials (RCTs) conducted in clinical research settings, the prevalence of Stage 1 TRD, defined as failure to achieve response after 1 course of adequate treatment, is approximately 50%, and the prevalence of Stage 2 TRD, defined as failure to achieve response after 2 courses of adequate treatment, is approximately 35%.1 Using these estimates and considering the 12-month prevalence of major depressive disorder (6.6%), the 12-month prevalence for Stage 1 and Stage 2 TRD in the population is approximately 3% and 2%, respectively.1 No estimate is available for Stages 3–5 TRD.1
Repetitive transcranial magnetic stimulation (rTMS) has been investigated as a noninvasive clinical tool to treat people with major depression. It has been proposed that major depression involves dysregulation of cortical activity, with lower activity in the left dorsolateral prefrontal cortex (dlPFC) and higher activity in the right dlPFC.2 High- and low-frequency magnetic stimulation seem to have opposite effects on cortical excitability. High-frequency stimulation increases and low-frequency stimulation decreases cortical excitability.2 There are many variations in the way rTMS can be applied, including unilateral or bilateral stimulation, choices of stimulation site, technical parameters and treatment duration. During the last 2 decades, more studies have used high-frequency rTMS (> 1 Hz) delivered to the left dlPFC than low-frequency stimulation (1 Hz) delivered to the right dlPFC. An alternative approach that was developed more recently is bilateral stimulation that targets both the left and the right dlPFC, performed either sequentially or simultaneously. 3 Some studies have used rTMS as an accelerating (add-on) strategy to antidepressant medications, and some have used it as a stand-alone treatment.
During the last 2 decades, a large number of sham-controlled trials investigated different methods of administering rTMS, including unilateral high-frequency, unilateral low-frequency and bilateral stimulation, using a variety of technical parameters. Meta-analyses conducted to date vary in many ways based on the nature of the research questions they aimed to address and on the research methods that they used. Most published systematic reviews have answered questions concerning the overall effect of the technique in patients with depressive disorders and synthesized the data collected from a variety of studies that were not limited to a specific population, or they combined data from different rTMS methods with different mechanisms of action and neurobiological basis. This mix of studies could further complicate the generalizability of the findings to a specific population and/or intervention. With a more defined target population and more stringent inclusion criteria, the results will be more applicable and clinically relevant to the population of interest. Therefore, the main goal of the present study was to determine the magnitude of the treatment effect of high-frequency unilateral rTMS and bilateral rTMS in patients with unipolar TRD, so that the results could be useful for clinical practice and inform the decision in the context of what to expect from each method of rTMS treatment in these patients. In addition, we aimed to assess whether the magnitude of the treatment effect differs when rTMS is used as an add-on strategy to antidepressant medications as opposed to a stand-alone treatment, and whether specific technical parameters are associated with better outcomes. We hypothesized that both unilateral and bilateral rTMS are effective in reducing depression scores and are superior to a sham treatment condition. Contrary to most previous meta-analyses, in which categorized response and remission data were chosen as the primary outcome, our approach was to examine the entire data set on a continuous scale and to control for the baseline depression scores.
We focused on the effect of rTMS in patients with TRD because patients with hard-to-treat depression represent a challenge to psychiatric and primary care clinics and are the patients most likely to be considered for nonpharmacological interventions such as rTMS. Despite growing interest in nonpharmacological options for people with TRD, we identified only 3 published meta-analyses, including 1 by our group, that specifically examined the treatment effect of rTMS in this population.4–6 However, in 2 of the meta-analyses, the pooled estimate was driven by inclusion of studies that performed unilateral high-frequency, unilateral low-frequency and bilateral stimulation, and in 1 study, no comparison was made between bilateral rTMS and sham treatment.
rTMS technique
In rTMS, an electromagnetic coil delivers multiple stimuli in trains, where each train consists of a number of pulses to be delivered. There must be an interval of no stimulation between the trains for safety reasons. Pulse duration is another key parameter that needs to be determined for each rTMS algorithm in consideration of other stimulation parameters. Guidelines on the safe use of rTMS have set a limit for a combination of stimulation parameters to prevent the occurrence of seizure or syncope.7,8 The occurrence of seizure with rTMS is extremely uncommon, but can be a serious adverse effect if the rTMS safety guideline is not followed, especially in patients being treated with drugs that potentially lower the seizure threshold.7
Although rTMS studies have used different treatment algorithms and different technical parameters, for positioning the coil on the scalp they commonly used the “5 cm rule,” which is 5 cm anterior to the point at which the motor threshold (MT) has been obtained. Only a few studies used neuronavigational systems to locate the area for stimulation. The reliability of a conventional 5 cm rule has been investigated in several neuronavigational studies,9–11 and it has been suggested that the current method for locating the dlPFC for stimulation using the area at which the MT is obtained is not precise and needs to be improved by neuronavigational systems. Stokes and colleagues12 have shown that rTMS protocols that do not account for individual variation in scalp to cortex distance take the risk of substantial under- or over-stimulation. They found that in their patients, the distance from the scalp to the dlPFC was 1–4.5 mm greater than the distance from scalp to motor cortex. They showed that every millimetre increase in the distance between the coil and prefrontal cortex requires about a 3% increase in intensity to produce equivalent neural response, but they also found this amount of increase would be in contrast with the rTMS safety guidelines. Although it would be very helpful to compare the outcomes of rTMS treatment with the use of neuronavigational systems versus the conventional method, the body of evidence for the use of neuronavigational systems is still too small to provide adequate power for analysis.
Methods
We developed a systematic review protocol a priori. In addition to the processes described in this article, our process also included scoping studies, reviewing related guidelines, reviewing prior systematic reviews, consultation with experts, contacting study investigators for additional information, and a team-based approach. Our main goal was to determine the magnitude of the treatment effect of high-frequency unilateral rTMS and bilateral rTMS in patients with unipolar TRD. We planned to examine the data on a continuous scale in order to preserve all the available information in each study as much as possible and to control for baseline values. We considered remission and response rates as our secondary outcomes. We stratified data according to antidepressant use during the trials to observe the magnitude of the effect in each stratum. We also performed sensitivity analysis, subgroup analysis, meta-regression analysis and publication bias analysis. We reported the rate for the most frequently observed adverse events during the trials.
Literature search strategy
We searched the literature using the Ovid interface in the following databases: MEDLINE, EMBASE, PsycINFO, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, and NHS Economic Evaluation Database. We used the following terms and subject headings: “depressive disorder,” “major depression,” “treatment-resistant depression,” “TRD,” transcranial magnetic stimulation” and “rTMS.” Our initial search was from inception up to Nov. 20, 2014, and we subsequently updated the search for new publications up to Apr. 3, 2017. We checked the reference lists of identified studies and prior meta-analyses as a supplement to our electronic searching, but did not identify any additional study that met our inclusion criteria.
Study selection process
We identified RCTs that were published in English and investigated the efficacy of unilateral or bilateral rTMS in adult patients who did not respond to treatment with antidepressant medications. We included studies of unilateral rTMS if they applied high-frequency rTMS to the left dlPFC as well as studies of sequential bilateral rTMS that applied low-frequency rTMS to the right dlPFC and high-frequency rTMS to the left dlPFC. We included only studies that investigated the effect of rTMS in patients with unipolar TRD. If studies included a few patients with bipolar disorder, we included them only if the proportion of these participants was 20% or less of the sample. The reason for excluding studies that included patients with bipolar disorder was that unipolar and bipolar disorders are separate diagnostic entities and, in DSM-5, a division has been made to separate bipolar disorders from depressive disorders. We included studies if participants received only 1 treatment session per day and had at least 10 sessions. We included studies reporting depression scores using the Hamilton Rating Scale for Depression (HAM-D).
We did not include novel rTMS interventions because the results of a network meta-analysis of different techniques13 did not support the efficacy of novel rTMS techniques compared with other rTMS interventions in treating depression. We excluded studies that evaluated the effect of rTMS on cognitive function, studies that evaluated the effectiveness of rTMS in depression due to specific conditions (i.e., poststroke depression, postpartum depression), studies that did not report the important outcomes for this review or provided insufficient data, and studies that exceeded the maximum allowed stimulation parameters set by the safety guidelines for rTMS. These guidelines7,8 indicate that all rTMS studies should proceed only with stringent safety measures and limits on stimulation parameters to avoid inducing seizure. In addition, we did not want to introduce bias toward the treatment effect estimate due to overstimulation.
Data abstraction
All abstracts were evaluated by 2 review authors independently, and the full texts of studies meeting the eligibility criteria were also reviewed in detail by the 2 review authors. The 2 review authors independently abstracted the relevant clinical data of the studies. Any disagreement about inclusion or data abstraction was resolved by consensus, following feedback received from a third team member.
Quality assessment
We examined the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework14 to show the confidence that might be placed on the body of evidence. The GRADE framework rates the confidence in the estimate of effect across the body of evidence for each outcome. The GRADE framework classifies the quality of evidence for each outcome into high, moderate, low, or very low categories using a step-wise, structural method.14
Statistical analysis
We used STATA software version 11 (StataCorp LP) to carry out the data analysis. Our primary outcome was change in depression scores from baseline, and our secondary outcomes were remission and response rates.
We stratified data based on whether rTMS was used as an add-on to antidepressant medications or as a stand-alone treatment. We calculated changes in mean depression scores from baseline to the end of the treatment for each study and meta-analyzed the data to produce a summary effect estimate represented by the weighted mean difference (WMD). For interpretation of WMD, if the 95% confidence interval (CI) does not include 0, the results are statistically significant at a 5% significance level. We also calculated the standardized mean difference (SMD) as another measure for magnitude of the treatment effect. We used Cohen’s conventional definition of small, medium and large SMD as 0.2, 0.5, and 0.8, respectively. Cohen has interpreted these numbers in terms of the percentage of nonoverlap of the treated group’s scores with those of the control group; an SMD of 0.8 indicates a nonoverlap of 47.4%, an SMD of 0.5 indicates a nonoverlap of 33%, and an SMD of 0.2 indicates a nonoverlap of 14.7% in the 2 distributions.
We assessed the degree of statistical heterogeneity among the studies using I2 statistics. Higgins and colleagues15 have proposed a tentative classification of I2 values and have assigned adjectives of low, moderate and high to the I2 of 25%, 50% and 75%. We tested the assumption of normality of data and constructed a Q–Q plot. The points were fairly close to the diagonal straight line, which was consistent with a normal distribution and used a random-effects model for meta-analyses.
To assess the robustness of our findings and to ensure similarity of the results across 2 time periods in which rTMS studies were conducted, we performed a sensitivity analysis based on the earlier and more recent studies. We performed subgroup analysis for rTMS technical parameters (frequency of stimulation, intensity of stimulation and number of treatment sessions) to see whether these intervention characteristics are associated with treatment efficacy. We categorized data into frequencies of 20 Hz and < 20 Hz, intensities of > 100% MT and ≤ 100% MT, and number of treatment sessions (≥ 15 and < 15). We also performed meta-regression analysis to examine the relationship between intervention characteristics and treatment effect. The p value of each regression coefficient indicates whether any particular variable has a significant effect.
For the secondary outcomes, we calculated pooled remission rates and pooled response rates as well as corresponding pooled rate ratios and rate differences. For relative measures, if the 95% CI does not include 1, the results are statistically significant at the 5% significance level. We preferred to report outcomes on the basis of intention to treat. To ensure that the studies included in the meta-analysis are not a biased sample of all relevant studies, we examined the possibility of publication bias and constructed a funnel plot for the primary outcome. In addition, we performed statistical tests for the presence of publication bias by calculating the fail-safe N and using Egger’s method.
Results
The database search yielded 3148 citations that were published from inception to Apr. 3, 2017. After examining the full text of the selected abstracts, 23 RCTs met the inclusion criteria. Nineteen studies compared unilateral rTMS with sham treatment, from which 3 assigned participants into 3 groups of bilateral, unilateral and sham treatment. Another 4 studies compared bilateral rTMS with sham treatment. The PRISMA flow chart shows the results of screening abstracts and full text citations (Appendix 1, Figure S1, available at jpn.ca/180056-a1).
Study quality
We examined the risk of bias in individual studies with respect to the allocation concealment, blinding, complete accounting of patients, and outcome reporting. For unilateral versus sham RCTs, only a few studies reported allocation concealment,16–18 but blinding of the participants and the outcome assessors were performed in all studies. Information regarding allocation concealment was unclear in the rest of the studies (84%) and, because of this limitation, we rated down for risk of bias as it may have compromised randomization. With further assessment of the other elements in the GRADE framework (inconsistency, indirectness, imprecision and publication bias), the GRADE for the body of evidence for unilateral versus sham RCTs was determined to be moderate. In the RCTs evaluating bilateral rTMS versus sham treatment, 3 of 7 RCTs performed allocation concealment,19–21 though blinding of the participants and the outcome assessors was done in all studies. Therefore, we did not rate down for risk of bias and determined the grade of evidence to be high (Appendix 1, Table S1).
Demographic characteristics
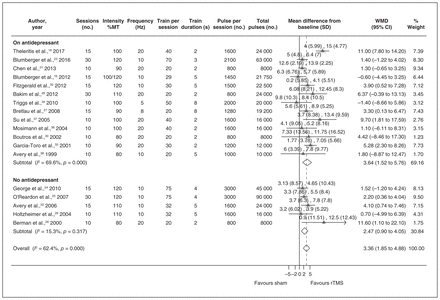
Table 1 shows study and participant characteristics for trials that compared unilateral and bilateral rTMS with sham treatment.
Study and participant characteristics of randomized controlled trials of unilateral and bilateral repetitive transcranial magnetic stimulation versus sham treatment
Unilateral rTMS versus sham treatment
Six of the 19 included studies reported statistical power of 80% or more to detect the true difference between the groups.17,18,22–25 The mean depression scores at baseline ranged from 20.5 to 40.8 in the rTMS group and from 19.5 to 37.3 in the sham group. While in 15 studies (79%) participants had Stage 2 TRD, 4 studies also included participants with Stage 1 TRD.24–27 In 14 studies (74%), participants received rTMS while receiving antidepressants, and in 5 studies participants did not receive any antidepressants during the rTMS treatment.22,24–26,28
Fourteen of the studies (74%) did not include participants with bipolar disorder.16,18,22–25,27–34 In studies that did include individuals with bipolar disorder, the proportion of those individuals ranged from 1.7% to 16.7%. The proportion of participants with bipolar disorder in the total sample was very small (0.9%). Two studies reported including participants with depression who also had psychosis, but the proportion of those with psychosis was low (5% and 7%).17,26
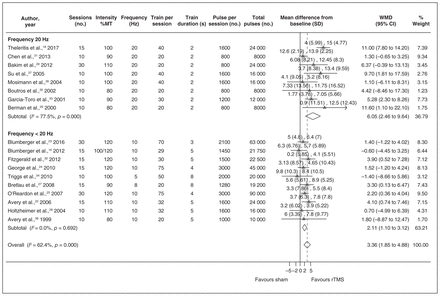
Bilateral rTMS versus sham treatment
The mean depression scores at the baseline ranged from 22.5 to 28.8 in the bilateral group, from 19.8 to 29.1 in the sham group, and from 23.7 to 26 in unilateral group. Participants in all studies had Stage 2 TRD. In 6 studies participants received rTMS with concomitant use of antidepressant medications, and in only 1 study35 participants did not receive antidepressants during rTMS treatment. Five studies reported including no patients with bipolar disorder,16,20,21,23,32 and 2 reported that the proportion of patients with bipolar disorder was 16% or less.19,35 The 2 studies that included patients with bipolar disorder did not report depression scores and were not included in the calculation of WMD. The proportion of patients with bipolar disorder for calculation of remission or response rates was 5.5% or less. Two studies reported that they did not include patients with psychosis.21,35
Characteristics of interventions
Unilateral rTMS versus sham treatment
Five studies used neuroimaging systems to localize the dlPFC site of stimulation more reliably.18,22–24,32 The remaining studies used the 5 cm rule, defined as the region 5 cm anterior to the site at which the MT for stimulation of the abductor pollicus brevis muscle was obtained. The frequency of stimulation across the studies ranged from 5 to 20 Hz, and the intensity of stimulation was between 80% and 120% of participants’ MT. Seven studies used a stimulation intensity of more than 100% of MT.22–25,28,29,32 The number of trains per session ranged from 20 to 75, and the train duration ranged from 2 to 8 s. The intertrain interval varied across the studies, ranging from 22 to 60 s. Two studies did not report the intertrain interval.31,32 The number of sessions varied from 10 to 30, and the number of pulses per session ranged from 800 to 3000. The total number of pulses during the entire rTMS treatment ranged from 8000 to 90 000.
Sixteen studies reported that the rTMS coil shape was a figure-eight.16–18,22,23,26–34,36,37 The sham condition in most of these studies was created using the same active coil held on the scalp with an angle and not tangentially. Nine studies held the coil at 90°,16,18,22,23,30,31,33,36,37 and 5 studies held it at 45°.26,28,29,32,38 This method was used to blind the participants to the type of intervention they were receiving, as the sham coil sounded like a real condition, but with no ensuing stimulation of the cortical structures. Three studies used an embedded magnetic shield to prevent the magnetic energy from reaching the cortex,24,25,34 and 2 studies used either a placebo coil or a coil that had no electric connection.17,27
Bilateral rTMS versus sham
Seven studies compared bilateral rTMS with sham, of which 3 studies16,23,32 also compared bilateral rTMS with unilateral rTMS. Two studies23,32 used neuroimaging systems to localize the dlPFC site of stimulation more reliably, and 5 studies used the 5 cm rule. Frequency of stimulation to the left dlPFC for both bilateral and unilateral rTMS groups was 10 Hz in 6 studies and 20 Hz in 1 study.20 Frequency of stimulation to the right dlPFC was 1 Hz in all bilateral rTMS studies. The intensity of stimulation ranged from 100% to 120% MT for both bilateral and unilateral rTMS groups. In bilateral rTMS groups, the number of trains per session ranged from 15 to 50 for the left dlPFC and from 1 to 30 for the right dlPFC. In unilateral rTMS groups, the number of trains per session ranged from 29 to 70. The train duration ranged from 2 to 5 s for left dlPFC and from 60 to 900 s for the right dlPFC. The train duration for the unilateral rTMS groups ranged from 3 to 5 s. The number of sessions varied among the studies from 10 to 30 sessions.
The number of pulses per session in bilateral rTMS groups ranged from 750 to 1500 for the left dlPFC and from 420 to 1800 for the right dlPFC. The number of pulses per session in unilateral rTMS groups ranged from 1450 to 2100. The total number of pulses for the bilateral rTMS groups ranged from 7500 to 45 000 for the left dlPFC and from 4200 to 18 000 for the right dlPFC. The total number of pulses for the unilateral rTMS groups ranged from 22 500 to 63 000. The sham condition was created using the same active coil held on the scalp with a 45° angle19,20,32 or a 90° angle.16,23,35 One study used an inactive or placebo sham condition in which no energy was delivered to the coil.21
Reported outcomes
Most studies reported depression scores at baseline and at the end of treatment. Response rate was defined as 50% or more reduction in depression scores, but studies used different thresholds to define remission, ranging from scores of 7 or less to scores of 10 or less. Among studies that compared unilateral rTMS with sham treatment, 1 study29 investigated the effectiveness of 2 types of intensity (80% MT and 110% MT), and another study37 investigated the effectiveness of 2 types of frequency (5 Hz and 20 Hz). For calculation of the mean difference, we included only the higher frequency or intensity reported by these studies to avoid duplication of data in the analysis. However, for calculation of remission and response rates, we included all data from the 2 studies.
Analysis of the primary outcome
Unilateral rTMS versus sham treatment
Eighteen studies reported on the mean depression scores before and after treatment. We meta-analyzed the data on the changes in mean depression scores from baseline to the end of treatment. The pooled estimate of WMD was 3.36 (95% CI 1.85–4.88, I2 = 62.4%). The WMDs for studies with and without concomitant use of antidepressants were 3.64 (95% CI 1.52–5.76, I2 = 69.6%) and 2.47 (95% CI 0.90–4.05, I2 = 15.3%), respectively (Fig. 1). The SMD was 0.50 (95% CI 0.28–0.73, I2 = 58.2%). The SMDs for studies with and without concomitant use of antidepressants were 0.58 (95% CI 0.25–0.91, I2 = 63.1%) and 0.29 (95% CI 0.12–0.47, I2 = 7.1%; Appendix 1, Fig. S2)
Weighted mean difference in depression scores in unilateral high-frequency repetitive transcranial magnetic stimulation versus sham treatment. CI = confidence interval; MT = motor threshold; rTMS = repetitive transcranial magnetic stimulation; SD = standard deviation; WMD = weighted mean difference.
Sensitivity analysis
We performed a sensitivity analysis considering the 2 time periods in which the studies were published (before 2010 and 2010–2017). The WMDs for the 2 time periods were very close (before 2010: WMD 3.59, 95% CI 1.95–5.23, I2 = 15.6%; 2010–2017: WMD 3.03, 95% CI 0.70–5.36, I2 = 77.3%). We were confident that the results of the earlier studies did not influence the outcome.
Subgroup analysis
We stratified the data for rTMS technical parameters that we assumed might be associated with the treatment effect and carried out 3 subgroup analyses for frequency of stimulation, intensity of stimulation and number of treatment sessions. The subgroup analysis for the frequency of stimulation revealed a relatively larger treatment effect for studies that applied a frequency of 20 Hz (n = 8) than for studies that applied frequencies of less than 20 Hz (n = 10). The WMD for the subgroup of studies with the frequency of 20 Hz was 6.05 (95% CI 2.46–9.64, I2 = 77.5%), while it was 2.11 (95% CI 1.10–3.12, I2 = 0%) for the subgroup of studies that used frequencies of less than 20 Hz (Fig. 2).
Weighted mean difference in depression scores in unilateral high-frequency repetitive transcranial magnetic stimulation versus sham treatment, stratified by frequency of stimulation. CI = confidence interval; MT = motor threshold; rTMS = repetitive transcranial magnetic stimulation; SD = standard deviation; WMD = weighted mean difference.
Contrary to what one might expect, the subgroup analysis for the intensity of stimulation showed a smaller treatment effect for higher intensity. The WMD for intensity greater than 100% (n = 7) was 2.39 (95% CI 1.28–3.50, I2 = 0%), while it was 4.08 (95% CI 1.30–6.87, I2 = 0%) for intensity of 100% or less (n = 11). We noted that most studies using an intensity of 100% or less used a frequency of 20 Hz; therefore, we could not make any conclusion based on this subgroup analysis. The subgroup analysis for the number of treatment sessions showed a WMD of 3.51 (95% CI 1.43–5.59, I2 = 75.2%) for studies using 15–30 sessions (n = 9) and a WMD of 3.09 (95% CI 0.74–5.44, I2 = 36.5%) for studies using fewer than 15 sessions (n = 9). Of note, only 2 of the 9 studies using 15–30 sessions used a frequency of 20 Hz.
Meta-regression analysis
We used a meta-regression technique to examine the effect of rTMS technical parameters on the outcome of the treatment. We used the WMDs from each study as dependent variables and 4 rTMS technical parameters (frequency of stimulation, intensity of stimulation, train duration, number of treatment sessions) as predictor variables. We used the restricted maximum likelihood as the method of estimation of between-study variance. The meta-regression analysis showed that intensity of stimulation, train duration and number of treatment sessions were not significant predictors of the treatment effect, whereas the frequency of stimulation could predict the treatment outcome (p = 0.04). We also performed a meta-regression analysis using number of total pulses, which is a product of several stimulation parameters. No fit was found in the model, and the graph showed no association between total number of pulses over the course of the treatment and the treatment effect (Fig. 3).
Meta-regression line plot for weighted mean differences over total pulses in unilateral high-frequency repetitive transcranial magnetic stimulation versus sham treatment. The estimate from each study is represented by a circle. Circle sizes vary with inverse of within-study variance. WMD = weighted mean difference.
Bilateral rTMS versus sham treatment
Four studies reported on the depression scores before and after treatment.16,20,23,32 One additional study19 reported only the percentage of improvement in mean depression scores using the HAM-D. Therefore, we did not have the data to include this study in the calculation of WMD. However, it seems that the percentage of the change from baseline in the bilateral group was a great improvement (mean 45.2% ± standard deviation [SD] 40.1%). The WMD between bilateral rTMS and sham treatment was 2.67 (95% CI 0.83–4.51, I2 = 0%; Fig. 4) and the SMD was 0.42 (95% CI 0.13–0.71, I2 = 0%). All but 1 study35 used bilateral rTMS with concomitant use of antidepressants. Therefore, we were not able to stratify the data according to this variable. In addition, we did not have a sufficient number of studies to perform subgroup and meta-regression analyses.
Weighted mean difference in depression scores in bilateral sequential repetitive transcranial magnetic stimulation versus sham treatment. CI = confidence interval; MT = motor threshold; rTMS = repetitive transcranial magnetic stimulation; SD = standard deviation; WMD = weighted mean difference.
The WMD between bilateral rTMS and unilateral rTMS was 0.97 (95% CI −2.71 to 4.65, I2 = 67.8%) and the SMD was considered to be small (0.12, 95% CI −0.39 to 0.63, I2 = 63.7%). There was no significant difference between the 2 groups. However, because the comparison between bilateral rTMS and unilateral rTMS was not included in our hypotheses and we did not intend to include all studies that compared these 2 techniques, we cannot draw any conclusion based on this comparison.
Analysis of secondary outcomes
Unilateral rTMS versus sham treatment
Seventeen studies reported on the response rate, but only 13 studies reported on the remission rate. The pooled remission rates for unilateral rTMS and sham groups were 16.0% and 5.7%, respectively. The remission rate for the unilateral rTMS group was 17.5% when rTMS was used with antidepressants and 15.1% when it was used as a stand-alone treatment. The pooled response rates for unilateral rTMS and sham groups were 25.1% and 11.0%, respectively. The pooled response rate for the unilateral rTMS group was 29.3% when rTMS was used with antidepressants and 21.4% when it was used as a stand-alone treatment. The pooled rate ratios for remission and response were 2.33 (95% CI 1.52–3.58, I2 = 0%) and 2.00 (95% CI 1.26–3.19, I2 = 50.4%), respectively, favouring unilateral rTMS (Appendix 1, Figs. S3 and S4). The rate differences between unilateral rTMS and sham treatment for remission and response outcomes were 0.09 (95% CI 0.04–0.14, I2 = 51.2%) and 0.13 (95% CI 0.05–0.2, I2 = 66.1%), respectively. The numbers needed to treat for remission and response were 11 and 8, respectively, meaning that if 11 patients were treated with unilateral rTMS, 1 would have a chance of remission.
Bilateral rTMS versus sham treatment
All 7 studies reported on the response rate, and 6 of them also reported on the remission rate. The pooled remission rate for bilateral rTMS and sham groups were 16.6% and 2.0%, respectively. The pooled response rates for bilateral rTMS and sham groups were 25.4% and 6.8%, respectively. The pooled rate ratios for remission and response rates were 5.54 (95% CI 1.96–15.61, I2 = 0%) and 3.55 (95% CI 1.87–6.76, I2 = 0%), favouring bilateral rTMS (Appendix 1, Figs. S5 and S6). The rate differences between bilateral rTMS and sham groups for remission and response outcomes were 0.13 (95% CI 0.01–0.25, I2 = 78.8%) and 0.17 (95% CI 0.06–0.28, I2 = 57.8%). The numbers needed to treat for remission and response were 8 and 6, respectively, meaning if 8 patients were treated with bilateral rTMS, 1 would have a chance of remission.
The pooled rate ratios for remission and response rates comparing bilateral rTMS with unilateral rTMS were 3.59 (95% CI 1.25–10.37, I2 = 0%) and 2.57 (95% CI 0.81–8.13, I2 = 26.6%), respectively. However, because the comparison between bilateral rTMS and unilateral rTMS was not included in our hypotheses and we did not intend to include all studies that compared these 2 techniques, we cannot draw any conclusion based on this comparison.
Publication bias analysis
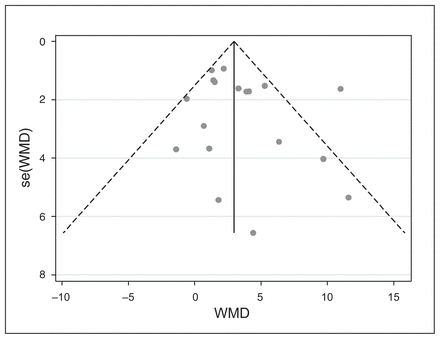
Publication bias occurs when the published studies are a biased sample of all relevant studies that were conducted. If studies with statistically significant results were given more chance to be published than studies with negative or nonsignificant findings, then the sample of included studies in the meta-analysis would be biased, compromising the quality of the evidence and the confidence that one can place on the estimate of the treatment effect. One method of assessing publication bias is constructing a funnel plot, as publication bias is one of the reasons that can cause asymmetry in the plot. We constructed a funnel plot for studies that compared unilateral rTMS with sham treatment based on the WMD in depression scores to see whether small studies with negative or nonsignificant results were missing from the plot. We visually inspected the plot for the presence of asymmetry and a gap where unpublished nonsignificant studies would have been. We observed that smaller studies with larger standard errors were scattered widely at the bottom of the plot, while the spread narrowed among larger studies at the top of the plot. Visual inspection of the plot did not show any evidence of publication bias (Fig. 5).
Funnel plot of standard error by weighted mean difference in unilateral high-frequency repetitive transcranial magnetic stimulation versus sham treatment. The circles correspond to individual studies. SE = standard error; WMD = weighted mean difference.
Because the interpretation of the funnel plot may be subjective and therefore open to different opinions, we used 2 statistical tests (fail-safe N, Egger test) to ensure the validity of our interpretation of the funnel plot. The fail-safe N test showed that 70 missing studies with an effect size of zero would be necessary to reduce the overall effect size to 0.1. The Egger test produced an estimated bias coefficient of 0.781 with a standard error of 0.815 and p = 0.352. The 2 statistical tests supported the robustness of the funnel plot against publication bias. We did not have a sufficient number of bilateral rTMS versus sham trials to construct a funnel plot for publication bias.
Adverse events
All but 1 study31 reported on the occurrence of adverse events. No seizures were reported. A variety of adverse events were reported with varying rates across studies. The most frequently occurring adverse events were headache (rTMS: 0%–60%; sham: 0%–50%), scalp pain or discomfort (rTMS: 4.5%–79%; sham: 0%–21%), gastrointestinal problems (rTMS: 5%–22%; sham: 0%–22%), eye problems (rTMS: 5.6%– 21%; sham: 0%–1.9%), muscle twitching (rTMS: 0%–20.6%; sham: 0%–3.2%), vertigo or dizziness (rTMS: 0%–16.7%; sham: 2%–14%), insomnia (rTMS: 4.5%–7.6%; sham: 0%–10%) and tinnitus (rTMS: 0%–11%; sham: 0%–3%).
Discussion
The aim of the present meta-analysis was to determine the magnitude of the treatment effect of unilateral high-frequency and bilateral rTMS in reducing depression symptoms in patients with unipolar TRD, who are most likely eligible for nonpharmacological intervention. With the restriction of the number of patients with bipolar disorder (i.e., up to 20%), the proportion of people with bipolar disorder in unilateral versus sham studies included in our analysis was only 0.9%. This small proportion is unlikely to have influenced the outcomes. Of the studies that compared bilateral rTMS with sham treatment, 2 studies included patients with bipolar disorder but did not report on depression scores and were not included in the calculation of WMD. We kept our analysis specific to clinical trials of rTMS that complied with rTMS safety guidelines to avoid introducing bias due to overstimulation and to ensure the applicability of the results to clinical practice. With respect to the method of analysis, we chose changes in depression score for our primary outcome. We did not choose dichotomized data (response and remission) as our primary outcome because dichotomization of continuous data could result in loss of information and statistical power and the possibility of type I error.39–41 In addition, it may happen that individuals close to but on opposite sides of the cut point are considered very different rather than very similar, which is clinically counterintuitive.41 Transforming the depression scores into the categories of response or no response results in the perception that the efficacy is either totally present or totally absent. However, some systematic reviews have reported only on remission and response, as these may be more easily communicated to physicians and patients.
Our study differs from 3 previous meta-analyses on patients with TRD.4–6 We included several new studies that were published after their search dates. Two of the previous meta-analyses5,6 combined the data from studies on high-frequency, low-frequency and bilateral stimulation, and 1 study4 did not compare bilateral rTMS and sham treatment. We have examined the effect of unilateral and bilateral rTMS separately, as these 2 techniques have different mechanisms of action and neurobiological basis. In addition, we have stratified the data to examine the performance of rTMS as an add-on and as a stand-alone treatment. This type of stratification was not applied in previous meta-analyses.
Our meta-analysis for the primary outcome for unilateral and bilateral rTMS showed that the WMDs in depression scores were statistically significant, in favour of rTMS. The WMD was 3.36 (95% CI 1.85–4.88) with unilateral rTMS and 2.67 (95% CI 0.83–4.51) with bilateral rTMS. We performed sensitivity analysis and tests for publication bias to investigate the robustness of our results for unilateral rTMS versus sham studies. Our sensitivity analysis confirmed the robustness of our findings and the publication bias analysis did not find any evidence that the pooled estimate of the treatment effect may have been influenced by unpublished studies. Although we have determined the magnitude of the treatment effect in patients with TRD, there are no validated, published criteria for minimum clinically important difference for this outcome. In 2004, the National Institute of Health and Clinical Excellence (NICE) guideline on treating depression suggested a difference of 3 points on the HAM-D and an SMD of 0.5 points to be clinically important differences, but no evidence was cited to support the proposed cut-off points. These points were criticized as being arbitrary and were subsequently removed in the 2009 edition of the NICE guideline.42 For unilateral rTMS, we stratified the data according to the use of antidepressant medications during the trials. It appeared that the magnitude of the treatment effect was relatively larger when rTMS was used in combination with antidepressant medications than when it was used as a stand-alone treatment. However, because participants were not randomized to these groups, this finding can be used only as a suggestion for future research.
Our study showed that with both unilateral and bilateral techniques, depression scores reduced by half in one-quarter of the participants. However, it appeared that participants who received sham rTMS also benefited, as 11% of sham participants in unilateral studies and 6.8% of sham participants in the bilateral studies also responded to the sham treatment. This may reflect the use of a suboptimal control condition in most studies by tilting the coil, which may have produced some cortical activity. The rate ratios for remission and response were both statistically significant in studies that compared unilateral or bilateral rTMS with sham treatment. We also calculated the rate difference for remission and response and respective numbers needed to treat. The numbers needed to treat for remission and response based on unilateral versus sham studies were 11 and 8, respectively, meaning if 11 patients were treated with unilateral rTMS, 1 would have a chance of remission. The numbers needed to treat for remission and response based on bilateral versus sham studies were 8 and 6, respectively, meaning if 8 patients were treated with bilateral rTMS, 1 would have a chance of remission.
Our subgroup analysis with respect to the rTMS technical parameters showed that the WMD for the subgroup of studies that used a frequency of 20 Hz was 6.05 (95% CI 2.46–9.64), while it was 2.11 (95% CI 1.10–3.12) for studies that used a frequency of 10 Hz or less. We found in our meta-regression analysis that the frequency of stimulation was a significant contributor to the treatment efficacy (p = 0.04). We were not able to perform a similar subgroup analysis for bilateral studies. However, because 6 of the 7 bilateral studies used a frequency of 10 Hz, it could be assumed that using a lower frequency in bilateral studies might have resulted in a relatively lower magnitude of effect in bilateral rTMS versus sham studies than the effect we observed in unilateral rTMS versus sham studies.
In the sample of studies that we included in this review, the WMD between unilateral and bilateral rTMS was not significant. However, we cannot make any conclusion based on this finding because such a comparison was not included in our hypotheses and would have required identifying all relevant published RCTs that compared the 2 modalities. However, our finding was in line with the results of other studies. A systematic review by Zhang and colleagues43 that included 10 RCTs that compared the 2 techniques in patients with TRD did not find any significant difference between the 2 modalities for remission and response rates. A systematic review by Chen and colleagues44 that included 7 RCTs but was not specific to patients with TRD also showed that unilateral and bilateral rTMS had comparable efficacy in treating major depression.
Recently, researchers have been more inclined to use neuronavigational systems in place of the conventional 5 cm method to locate the dlPFC for coil positioning. In the most recent rTMS study by Theleritis and colleagues,18 which produced a large treatment effect, a neuronavigational system was used to locate the target area. The investigators found the prefrontal cortex location in a more anterior position than the one located using the 5 cm rule. Avery and colleagues22 have also shown greater remission and response rates (20% and 30%, respectively) with the use of neuroimaging to adjust for coil-to-cortex variation among their participants. George and colleagues24 used MRI scans to adjust for coil placement only in one-third of their participants. More recently, Blumberger and colleauges23 used a cortical coregistration technique with structural MRI to localize the dlPFC. We were not able to analyze the data based on the use of neuronavigational systems because we did not have an adequate number of studies to test any hypothesis. However, with accumulation of data in the future, the use of neuronavigational systems may become more commonplace outside of the research environment, although implementation of these systems has associated costs, which can limit their use in clinical practice.
We compared the results of our meta-analysis of unilateral versus sham trials with prior meta-analyses on patients with TRD. A meta-analysis by Gayness and colleauges5 that updated the original systematic review by the Agency for Healthcare and Quality (AHRQ) in 2011 found a larger treatment effect (WMD 4.81, 95% CI 3.52–6.11). Their pooled remission rate for the unilateral rTMS group was 21% compared with 16% in our current study. However, their remission rate for the sham group (6%) was about the same as that in our study (5.7%). Their pooled response rates for rTMS and sham groups were 26% and 9%, respectively, compared with our pooled response rates of 25.1% for the rTMS group and 11.0% for the sham group.
The results of the meta-analysis by Lam and colleagues,6 which was conducted a decade ago, were very similar to those of the current study. They reported an SMD of 0.48 (95% CI 0.28–0.69), pooled remission rates of 17% and 6%, and pooled response rates of 25% and 9% for rTMS and sham groups, respectively. These 2 meta-analyses included patients with TRD but combined the results of studies on unilateral high-frequency, unilateral low-frequency and bilateral stimulation.
The results of a previous meta-analysis completed by several authors in our group4 showed a pooled WMD of 2.31 (95% CI 1.19–3.43), which was lower than the WMD in the current study. This difference is likely explained by the influence of a new publication by Theleritis and colleagues18 that showed an 11-point difference between unilateral rTMS and sham treatment. However, the remission and response rates for rTMS and sham groups were very close to the rates in the current study (remission: 17% for rTMS and 7% for sham; response: 25% for rTMS and 12% for sham).
We also attempted to compare the results of our analysis of bilateral versus sham trials with those of prior meta-analyses. The only meta-analysis that had inclusion criteria somewhat similar to those of the current study was the study by Berlim and colleagues.45 They found pooled remission rates of 19% and 2.6% and pooled response rates of 24.7% and 6.8% for bilateral rTMS and sham groups, respectively. These estimates were close to our findings (remission: 16.6% for rTMS and 2.0% for sham; response: 25.4% for rTMS and 6.8% for sham).
Limitations
We had limited data for further analysis on the patient-related prognostic variables and, therefore, we could not test whether the age of the patients or degree of treatment resistance with respect to the number of failed antidepressant trials have influenced the outcomes. However, most studies in our meta-analysis included participants with Stage 2 TRD (79% of unilateral rTMS versus sham and 100% of the bilateral rTMS versus sham trials). We were unable to assess whether bilateral rTMS has a different effect on patients who were not taking antidepressant medications because of the small number of trials and the fact that in 6 of the 7 trials patients received antidepressant medications.
A limitation that pertains to the design of the trials is that the sham coil used in most studies may not have been an ideal technique to create a control condition and may have induced magnetic stimulation. Because most studies used the tilting technique, it is possible that the active magnetic field entered the brain cortex of the participants in the control group, causing some treatment effect. It is also possible that the sham technique used in many studies was a suboptimal condition to blind the patients, jeopardizing the integrity of blinding.
Strengths
This meta-analysis best addresses the magnitude of the treatment effect of rTMS in patients with unipolar TRD. This study has several strengths. We used explicit criteria for TRD and stringent inclusion criteria for unipolar depression, we controlled for baseline depression scores, and we reported outcomes based on a continuous scale without the need for a cut-off point as well as on ratio measures that classify patients into groups. In addition, we detected no publication bias that would affect the study outcomes. We included only studies that complied with rTMS safety guidelines with respect to the combination of technical parameters to avoid bias due to overstimulation, and the information was obtained from moderate/high-quality evidence. Therefore, our estimate for magnitude of effect in unipolar TRD is more certain than what was reported in the previous meta-analyses.
In addition, we did not combine the effect of different rTMS protocols. Because patients with TRD who are eligible to receive rTMS will undergo 1 technique at a time, the results of this study are more relevant to daily clinical practice and real-life situations. Furthermore, we have stratified data according to the concomitant use of antidepressant medications, which is not reflected in the previous meta-analyses and may be useful for further investigation.
Conclusion
Our study suggests that rTMS has moderate antidepressant effects and appears to be promising in the short-term treatment of patients with unipolar TRD. However, it is not clear from this study whether the effects are sustained over time without using maintenance treatment. The full therapeutic potential of rTMS is yet to be discovered, and with accumulation of additional data, the role of technical parameters and the use of neuronavigational systems in improving the outcomes will become clearer. The evidence gathered over 2 decades of investigation gives optimism and hope that novel treatment strategies will be developed in the near future. Neuroimaging systems may broaden the understanding of the previous methodological issues and open avenues for further neuroscience research to optimize the technique and its full potential in treating patients with TRD.
Acknowledgements
The authors gratefully acknowledge and thank Caroline Higgins for conducting the literature search and creating the EndNote library.
Footnotes
Competing interests: S. Palimaka was employed by Janssen Canada from September 2015 to October 2016 and has been employed by Amaris, Inc. since February 2017. No other competing interests declared.
Contributors: S. Sehatzadeh, Z.J. Daskalakis, H.-A. Tu, S. Palimaka, J.M. Bowen and D.J. O’Reilly designed the study. S. Sehatzadeh, B. Yap and H.-A. Tu acquired the data, which S. Sehatzadeh and J.M. Bowen analyzed. S. Sehatzadeh wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Disclaimer: The conclusions expressed in this publication do not necessarily represent the opinions of Health Quality Ontario. No endorsement is intended or should be inferred.
- Received April 12, 2018.
- Revision received July 29, 2018.
- Accepted September 19, 2018.