Abstract
Background: Corticostriatal circuits (CSC) have been implicated in the presentation of some restricted and repetitive behaviours (RRBs) in children with autism-spectrum disorder (ASD), and preliminary evidence suggests that disruptions in these pathways may be associated with differences in genetic and environmental influences on brain development. The objective of this investigation was to examine the impact of genetic and environmental factors on CSC regions in twins with and without ASD and to evaluate their relationship with the severity of RRBs.
Methods: We obtained T1-weighted MRIs from same-sex monozygotic and dizygotic twin pairs, aged 6–15 years. Good-quality data were available from 48 ASD pairs (n = 96 twins; 30 pairs concordant for ASD, 15 monozygotic and 15 dizygotic; 18 pairs discordant for ASD, 4 monozygotic and 14 dizygotic) and 34 typically developing control pairs (n = 68 twins; 20 monozygotic and 14 dizygotic pairs). We generated structural measures of the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), caudate, putamen, pallidum and thalamus using FreeSurfer. Twin pair comparisons included intraclass correlation analyses and ACE modelling (a2 = additive genetics; c2 = common or shared environment; e2 = unique or nonshared environment). We also assessed correlations with RRB severity.
Results: Structural variation in CSC regions was predominantly genetically mediated in typically developing twins (a2 = 0.56 to 0.87), except for ACC white matter volume (a2 = 0.42, 95% confidence interval [CI] 0.08 to 0.77). We also observed similar magnitudes of genetic influence in twins with ASD (a2 = 0.65 to 0.97), but the cortical thickness of the ACC (c2 = 0.44, 95% CI 0.22 to 0.66) and OFC (c2 = 0.60, 95% CI 0.25 to 0.95) was primarily associated with environmental factors in only twins with ASD. Twin pair differences in OFC grey matter volume were also correlated with RRB severity and were predominantly environmentally mediated.
Limitations: We obtained MRIs on 2 scanners, and analytical approaches could not identify specific genetic and environmental factors.
Conclusion: Genetic factors primarily contribute to structural variation in subcortical CSC regions, regardless of ASD, but environmental factors may exert a greater influence on the development of grey matter thickness in the OFC and ACC in children with ASD. The increased vulnerability of OFC grey matter to environmental influences may also mediate some heterogeneity in RRB severity in children with ASD.
Introduction
Autism-spectrum disorder (ASD) is a prevalent neurodevelopmental disorder; recent estimates indicate that 1 in 40 to 1 in 59 children in the United States currently have a diagnosis.1,2 It is characterized by cognitive and behavioural impairments in multiple symptom domains, primarily social communication impairments and restricted and repetitive patterns of behaviour or interests (RRBs).3 The onset of these symptoms occurs early in life, typically within the first 3 years; however, diagnosable manifestations may not be observable until demands exceed an individual’s limitations. Symptoms of ASD are also extremely variable in presentation and severity across individuals and may originate from impairments across independent domains.4–6 Thus, there are likely multiple subgroups within the ASD population, in which different neurobiological mechanisms may underlie the development of different symptom domains across children with ASD.
Compared with social communication impairments, the RRB symptoms of ASD have received considerably less attention from research investigations. Studies to address this knowledge gap would be beneficial, because RRB symptoms (e.g., repetitive, sensory-motor actions) are present early in development7 and can cause significant management challenges and barriers to adaptive learning later in life.8 Preliminary neurobiological investigations suggest that corticostriatal circuits (CSC), which consist of frontal (orbitofrontal cortex [OFC] and anterior cingulate cortex [ACC]), striatal (caudate, putamen, pallidum) and thalamic regions, are of particular interest for the study of RRB in ASD because these networks are integrally involved with carrying out goal-directed behaviours, and disruption of these pathways is associated with aberrant behavioural presentations.9 For instance, CSC abnormalities are associated with compulsive grooming in mouse models of ASD, and manipulation of this circuit can modulate the severity of this behaviour.10 These findings are also consistent with neuroimaging investigations of CSC regions in people with ASD, which include reports of altered regional growth rates,11 reduced white matter quality,12 decreased functional activation and connectivity,13 and altered neurochemical levels.14 Most importantly, neurobiological alterations in CSC regions (e.g., ACC/OFC,15,16 striatum17,18 and thalamus19,20) have exhibited associations with the severity of RRB symptoms in clinical studies of people with ASD, suggesting that disruptions in CSC processing may mediate some variability in RRB presentation across children on the autism spectrum.
The heterogeneity of RRB symptoms could be associated with the variable effects of genetic and environmental influences on different CSC regions.10,21–24 Preliminary research using the ACE model (a2 = additive genetics; c2 = common or shared environment; e2 = unique or nonshared environment) to quantify the magnitude of genetic and environmental influences on global brain structures suggests that subcortical grey matter may be more genetically mediated in twins with ASD than in typically developing (TD) controls, whereas cortical grey matter, particularly thickness, may be more environmentally mediated.21 These observations suggest that differences in the impact of genetic susceptibility and prenatal/postnatal environmental exposures across CSC regions during development are related to the presentation of RRB in children with ASD. This potential relationship is also supported by animal models of RRB, in which environmental deprivation can increase RRB severity and alter brain development, whereas environmental enrichment can decrease severity and neurobiological alterations.10 To our knowledge, the influence of genetic and environmental factors on the development of CSC regions and RRB severity in children and adolescents with ASD has not yet been evaluated.
The objective of this investigation was to examine ASD-related differences in the influence of genetic25 and environmental26 factors on CSC regions in a sample of twins with and without ASD and evaluate their relationships with RRB severity across multiple behavioural subdomains. Based on previous investigations,27–29 we hypothesized that structural variation in the subcortical grey matter of CSC regions would be primarily genetically mediated in both twins with ASD and TD controls, potentially to a larger extent for twins with ASD, but that structural variation in the frontal grey matter of CSC regions would most likely be associated with environmental factors in only twins with ASD.21 We also expected that differences in RRB severity would be related to differences in the structural properties of CSC regions, such as between repetitive or compulsive behaviours and the grey matter volume of the striatum17,18 and OFC.16 Examining these relationships is important, because it will help account for additional variability in the heterogeneous neurobiological alterations and symptom presentations that are observed across individuals with ASD, which we hope will support advances in early identification and precision medicine.
Methods
Participants
Male and female same-sex twin pairs, in which at least 1 twin was diagnosed with ASD or both were TD, were recruited to participate in this study.21,23,30 Participants with ASD were recruited from the California Autism Twin Study31 and Interactive Autism Network Research Database. Additional ASD and TD twin pairs were also recruited from the local community using online and local advertisements. We confirmed ASD diagnosis with the Autism Diagnostic Interview–Revised (ADI-R)32 and the Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2).33 Any twin pairs in which either twin exhibited a genetic or metabolic disorder, an unstable medical condition (such as seizures), a history of traumatic head injury or asphyxia at birth or an MRI contraindication (such as ferrous implants) were excluded from this investigation. In addition, TD twin pairs were excluded if either twin exhibited a severe psychiatric disorder, a history of learning disabilities or a full-scale IQ of less than 70. These criteria were implemented to exclude potential confounding sources of variability in both ASD and TD twin pairs. After screening, 90 twin pairs (i.e., 180 individuals) were invited to participate. We confirmed zygosity from saliva samples based on 9 short tandem repeat loci and the X/Y amelogenin. Monozygotic twin pairs exhibited concordance on all markers, and dizygotic twin pairs were discordant for at least 1 marker.31 Informed consent was obtained from all parents and assent from all children. The methodology of this investigation was approved by the institutional review board.
Psychological testing
We examined general cognitive abilities using the Stanford–Binet Intelligence Scales, 5th edition,34 and assessed ASD-related impairments using the Social Responsiveness Scale (SRS)35 and Restricted Behaviour Scales–Revised (RBS-R),36 which provides subscale measures of multiple domains of repetitive behaviour. We measured handedness using the Edinburgh Handedness Inventory37 and quantified socioeconomic status using the Hollingshead method.38
Magnetic resonance imaging
We conducted MRIs on 2 identical GE 3T MR750 scanners at the same institution using a standard 8-channel head coil. Any ASD twins who were unable to lie still for scanning were administered propofol under the supervision of an anesthesiologist at 200–300 μg/kg/min to induce procedural sedation. We acquired 2 T1-weighted inversion recovery spoiled gradient recalled (IR-SPGR) echo pulse sequence images (188 coronal slices, repetition time 8.15 ms, echo time 3.24 ms, inversion time 600 ms, flip angle 12°, slice thickness 1.2 mm, field of view 22 × 22 cm, in-plane resolution 0.86 × 0.86, acquisition matrix size 256 × 192 mm, number of excitations = 1). The highest-quality image was visually identified and then analyzed with FreeSurfer v5.3.39 All automated procedures were inspected by trained raters, who corrected segmentation errors whenever possible; otherwise, the participant was excluded from subsequent analyses. We combined the left and right hemisphere labels from the Desikan–Killiany atlas40 to create regional measures of the OFC (lh_/rh_lateralorbitofrontal + lh_/rh_medialorbitofrontal) and ACC (lh_/rh_rostralanteriorcingulate + lh_/rh_caudalanteriorcingulate) for grey matter and white matter volume, as well as surface area. We also evaluated average grey matter thickness across labels for the OFC and ACC, and grey matter volumes of the left and right caudate, putamen, pallidum and thalamus labels were combined.
Two sets of twins were assessed on both scanners, and repeated-measures comparisons indicated an approximately 6% difference in total brain volume across sites Prior to Free-Surfer segmentation, affected scans (ASD and TD) were transformed using the FSL linear transformation package FLIRT41 with standard sinc interpolation to FSL standard orientation images using a matrix that was designed to minimize potential scanner effects across all global structural measures that were evaluated.21 Importantly, both twins from each pair were always scanned at the same location, and statistical analyses were based primarily on comparisons within twin pairs.
Statistical analysis
We generated intraclass correlation coefficients (ICCs) of structural data, adjusted for sex and diagnosis, using STATA42 and the DeFries–Fulker model43 for all monozygotic and dizygotic twin pairs to evaluate the general assumptions of twin modelling. Next, we examined the ICCs separately for the ASD and TD subgroups, adjusting for sex and excluding twin pairs discordant for ASD, and we compared them with Fisher z-transformation44 to provide an initial assessment for the magnitude of differences between diagnostic groups. Twin pairs discordant for ASD were excluded from these analyses because they may differ in environmental factors to a greater degree than concordant twin pairs, and our goal was to provide a more representative estimation of potential ASD-related differences in genetic and environmental influences on structural properties of the CSC regions. We then calculated the ACE model for broad sense heritability based on Falconer’s formula45 to estimate the contribution of genetic and environmental influences to structural variation in CSC regions. The ACE model estimates the proportion of variation in a trait of interest (e.g., OFC grey matter thickness or caudate grey matter volume) that is related to different factors (a2 = additive genetics; c2 = common or shared environment; e2 = unique or nonshared environment) by comparing trait variability in monozygotic versus dizygotic twin pairs who share many environmental features and differ in genetic influences by a known quantity (i.e., approximately 50%). We also assessed Pearson correlations of within-twin-pair differences in regional measures and symptom severity, as assessed with the SRS and RBS-R, to evaluate genetic and environmental influences on these brain–behaviour relationships. We used false discovery rate46 correction to account for multiple comparisons.
Results
Participants
Demographic and clinical characteristics of the twin samples can be found in Table 1.
Demographics and clinical characteristics*
Following confirmation of quality-assurance criteria, anatomic scans from 30 twin pairs in which both twins had ASD (15 monozygotic, 15 dizygotic), 18 twin pairs discordant for ASD (4 monozygotic, 14 dizygotic) and 34 TD twin pairs (20 monozygotic, 14 dizygotic) were available for analysis (n = 164 twins). Discordance for ASD was defined as 1 twin meeting diagnostic criteria on the ADI-R32 and ADOS-2,33 and the other twin not meeting criteria for either ASD or exhibiting subthreshold ASD-related impairments. Comparing concordant ASD (n = 30 pairs) and TD (n = 34 pairs) twin pairs, SES was slightly lower in ASD twin pairs than in TD controls (mean difference between groups [M] = −5.29, 95% CI −10.51 to −0.72), predominantly owing to dizygotic twin pairs (M = −10.06, 95% CI −18.11 to −2.01). We found no other zygosity × diagnostic group differences, and adjusting for SES did not significantly alter the twin modelling results, indicating that the groups were relatively well matched. Although we observed some within-diagnostic-group differences in SRS scores between dizygotic (ASD n = 58; TD n = 28) and monozygotic (ASD n = 38; TD n = 40) twins (ASD M = −7.39, 95% CI −14.26 to −0.13; TD M = −4.28, 95% CI −6.82 to −1.74), TD twins were below the threshold for any ASD-related impairments,35 and ASD subgroup differences were likely related to the larger number of dizygotic twin pairs discordant for ASD.
Comparison of CSC structural measures across diagnostic groups
Diagnostic group comparisons of regional structural data are included in the supplementary materials (Appendix 1, Table S1 and Table S2, available at jpn.ca/190030-a1). As expected, we observed only moderate ASD-related differences, which indicated larger grey matter volume of the striatal regions (putamen M = 431.81, 95% CI 9.95 to 853.67) but thinner cortical grey matter (OFC M = −0.07, 95% CI −0.13 to −0.004) in twins with ASD (n = 78) compared with TD controls (n = 68). Interestingly, thalamic grey matter volume was smaller (M = 1125.86, 95% CI 249.44 to 2002.28) and OFC grey matter was thicker (M = 0.12, 95% CI 0.03 to 0.20) in twins with ASD compared with unaffected co-twins (n = 18 pairs), suggesting that greater control over potentially confounding sources of variability (e.g., shared environmental influences) can provide additional insights into ASD-related alterations.
ICCs of CSC structural measures in monozygotic and dizygotic twin pairs
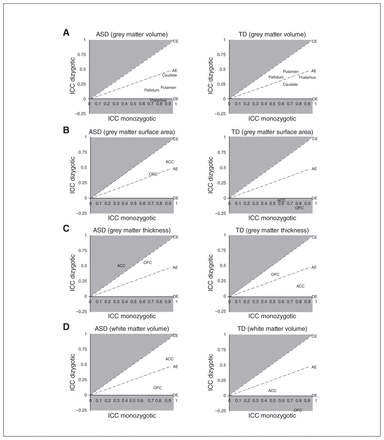
In all twin pairs, regardless of diagnosis, ICCs were positive, statistically significant and higher in monozygotic twin pairs (n = 78 twins/39 pairs) than in dizygotic twin pairs (n = 86 twins/43 pairs; p < 0.05 in most instances; Appendix 1, Table S3). The only exceptions were zygosity subgroup comparisons for the ACC, in which white matter volume (ICC monozygotic = 0.93, ICC dizygotic = 0.95, z = −1.09, p = 0.28), grey matter surface area (ICC monozygotic = 0.95, ICC dizygotic = 0.92, z = 1.52, p = 0.13) and cortical thickness (ICC monozygotic = 0.65, ICC dizygotic = 0.53, z = 1.16, p = 0.25) were not statistically different in monozygotic compared with dizygotic twins (see Discussion).
The ICC estimates for monozygotic ASD twin pairs (n = 30 twins/15 pairs) and TD twin pairs (n = 40 twins/20 pairs; Fig. 1; Appendix 1, Table S4) were also generally of large magnitude (approximately 0.50 to 0.90) and statistically significant (p < 0.01) in all instances, with the exception of ACC grey matter thickness in ASD twin pairs (ICC = 0.35, 95% CI −0.03 to 0.73) and white matter volume in TD twin pairs (ICC = 0.49, 95% CI 0.013 to 0.84). Comparing monozygotic twins across groups, ICCs were larger in monozygotic ASD twin pairs compared with monozygotic TD twin pairs for ACC white matter volume (ICC ASD = 0.91, ICC TD = 0.49, z = 3.92, p < 0.001) and grey matter surface area (ICC ASD = 0.91, ICC TD = 0.60, z = 3.30, p = 0.001), as well as grey matter volume of the caudate (ICC ASD = 0.91, ICC TD = 0.69, z = 2.68, p = 0.01) and putamen (ICC ASD = 0.90, ICC TD = 0.71, z = 2.31, p = 0.02). Conversely, the ICC for ACC grey matter thickness was larger in monozygotic TD twins compared with monozygotic ASD twins (ICC ASD = 0.35, ICC TD = 0.81, z = −3.01, p = 0.003). These differences were generally of large magnitude and survived correction for multiple comparisons.
Intraclass correlations in ACE model space (A = additive genetics; C = common or shared environment; E = unique or nonshared environment; D = genetic dominance). Intraclass correlation coefficients within twin pairs, in which both twins were diagnosed with autism-spectrum disorder (ASD0 or were typically developing (TD) controls (adjusted for sex), were generated within monozygotic and dizygotic twin pairs (ASD: monozygotic n = 15 pairs, dizygotic n = 15 pairs; TD monozygotic n = 20 pairs, dizygotic n = 14 pairs) and displayed in relation to ACE model space. Brain structures near or above the CE line (shaded) are primarily environmentally mediated, whereas brain structures near or below the DE line (shaded) are primarily genetically mediated. This figure provides a visualization of the data used to generate ACE model parameter estimates. ACC = anterior cingulate cortex; ICC = intraclass correlation coefficient; OFC = orbitofrontal cortex.
The ICC estimates were generally of lower magnitude (approximately −0.25 to 0.60) for dizygotic ASD twin pairs (n = 30 twins/15 pairs) and TD twin pairs (n = 28 twins/14 pairs; Fig. 1; Appendix 1, Table S4) compared with monozygotic twin pairs, and only a subset was statistically significant and lower in the dizygotic compared with the monozygotic subgroups at p < 0.05. Dizygotic ASD twins exhibited higher ICCs than dizygotic TD twins for ACC white matter volume (ICC ASD = 0.59, ICC TD = 0.09, z = 2.12, p = 0.03) and ACC (ICC ASD = 0.59, ICC TD = −0.02, z = 2.51, p = 0.01) and OFC grey matter surface area (ICC ASD = 0.39, ICC TD = −0.16, z = 2.07, p = 0.04). The diagnostic group comparisons between dizygotic twin pairs did not survive correction for multiple comparisons, but differences across the monozygotic and dizygotic subgroups for the ACC may have contributed to the overall twin pair observations outlined above.
ACE modelling of CSC structural measures
The ACE model provides estimates for the proportion of variation in a trait of interest that is related to additive genetic factors (a2), common/shared environmental factors (c2) or unique (e2) environmental factors (Table 2). Under a primarily genetic AE model, the ICCs in monozygotic twins are approximately twice that of dizygotic twins, whereas under a primarily environmental CE model, ICCs in monozygotic and dizygotic twin pairs are nearly equal. Within TD twin pairs, all structural measures of CSC regions that could be modelled were best fit with an AE model and exhibited significant genetic contributions (a2 = 0.56 to 0.87). Anterior cingulate cortex and OFC grey matter surface area, as well as the OFC white matter volume, could not be fit due to negative ICCs in the dizygotic TD subgroup; however, variation in these measures also appeared to be primarily genetically mediated based on ICC comparisons. Anterior cingulate cortex white matter volume was also best fit with the AE model in TD twins, but the majority of variation was attributed to the environmental factor (a2 = 0.42). No structural measures from CSC regions were best fit with the CE model in TD twins.
ACE model parameter estimates for concordant ASD and TD twin pairs*
In ASD twin pairs, we found some significant deviations from the primarily genetically mediated contributions that were identified in TD controls. Grey matter and white matter volume, as well as the surface area of all CSC regions, were also best fit with the AE model, and primarily genetically mediated in ASD twin pairs (a2 = 0.65 to 0.97); however, ACC and OFC grey matter thickness were best fit with the CE model (c2 = 0.44 to 0.60). This observation suggests that environmental factors may predominantly contribute to cortical thickness of the ACC and OFC in twins with ASD. Thalamic grey matter volume could not be modelled in ASD twin pairs because of a negative ICC in the dizygotic subgroup, but ICC comparisons suggested that it was also primarily genetically mediated, consistent with a previous study that examined markers of neuronal integrity in this structure in twins with ASD.23
RRB symptomatology and its relationship with CSC regions
Twins from ASD pairs exhibited more severe RRB symptoms than TD control twins (SRS M = 22.56, 95% CI 18.50 to 26.63; RBS-R M = 17.86, 95% CI 13.25 to 22.47) across all RRB subdomains that were assessed, including the stereotyped (M = 3.13, 95% CI 2.33 to 3.92), self-injurious (M = 2.20, 95% CI 1.40 to 3.00), compulsive (M = 2.94, 95% CI 1.89 to 4.00), ritualistic (M = 3.19, 95% CI 2.23 to 4.14), sameness (M = 3.87, 95% CI 2.46 to 5.29) and restricted behaviours (M = 2.35, 95% CI 1.58 to 3.11) subscales of the RBS-R. Interestingly, we also found relevant differences in RRB severity in monozygotic twin pairs concordant for ASD (SRS M = 12.73, 95% CI 5.79 to 19.67; RBS-R M = 8.60, 95% CI 4.47 to 12.73), including stereotyped (M = 1.40, 95% CI 0.45 to 2.36), sameness (M = 1.47, 95% CI 0.29 to 2.65) and restricted behaviours (M = 1.67, 95% CI 0.59 to 2.75), but only moderate differences in compulsive (M = 1.80, 95% CI −0.18 to 3.78) and ritualistic behaviours (M = 1.00, 95% CI −0.25 to 2.03). These observations suggest that nongenetic factors may contribute to RRB severity in children and adolescents with ASD. The influence of nongenetic factors on RRB severity was further supported by ACE modelling in children and adolescents with ASD for sameness (c2 = 0.89, 95% CI 0.74 to 1.04, p < 0.001) and restricted behaviours (c2 = 0.53, 95% CI 0.15 to 0.91, p < 0.006), but stereotyped behaviours appeared to also have significant genetic contributions (a2 = 0.74, 95% CI 0.47 to 1.01, p < 0.001).
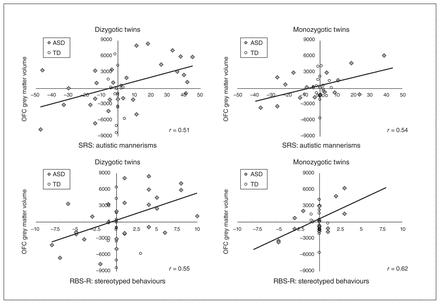
Correlations of twin pair differences in structural measures and symptom severity also indicated some interesting brain–behaviour relationships between CSC and RRB (Fig. 2). The primary associations identified were between differences in OFC grey matter volume and RRB severity in twins with ASD. Across all dizygotic twin pairs, regardless of diagnostic group, OFC grey matter volume was associated with the severity of autistic mannerisms (r = 0.43, 95% CI 0.14 to 0.65, p = 0.005), stereotyped behaviours (r = 0.44, 95% CI 0.15 to 0.66, p = 0.005) and compulsive behaviours (r = 0.38, 95% CI 0.08 to 0.61, p = 0.016). These relationships appeared to be primarily owing to dizygotic ASD twins (autistic mannerisms r = 0.51, 95% CI 0.17 to 0.74, p = 0.005; stereotyped behaviours r = 0.55, 95% CI 0.21 to 0.77, p = 0.003; compulsive behaviours r= 0.41, 95% CI 0.05 to 0.68, p = 0.029) and not TD controls (p > 0.05 in all instances for this group). We also observed similar relationships for monozygotic twin pairs, regardless of diagnostic group, for OFC grey matter volume and the severity of autistic mannerisms (r = 0.40, 95% CI 0.09 to 0.64, p = 0.013) and stereotyped behaviours (r = 0.43, 95% CI 0.13 to 0.66, p = 0.007), but not compulsive behaviours (r = 0.30, 95% CI −0.02 to 0.57, p = 0.07). These relationships also appeared to be primarily owing to monozygotic ASD twins (autistic mannerisms r = 0.54, 95% CI 0.10 to 0.80, p = 0.021; stereotyped behaviours r = 0.62, 95% CI 0.21 to 0.84, p = 0.006) and not TD controls (p > 0.05 in all instances). The similarity between monozygotic and dizygotic twin pairs with ASD suggests that environmental factors may also contribute to the relationship between CSC alterations and RRB severity in children and adolescents with ASD.
Relationships between twin pair differences in grey matter volume and severity of restricted and repetitive behaviours (RRB). Within-twin-pair differences in grey matter volume (mm3) of the orbitofrontal cortex (OFC) are displayed in relation to RRB, as assessed by the autistic mannerisms subscale of the Social Responsiveness Scale (SRS) and the stereotyped behaviours subscale of the Restricted Behaviour Subscales – Revised (RBS-R), for dizygotic and monozygotic twin pairs in which at least 1 twin was diagnosed with autism-spectrum disorder (ASD) or both were typically developing (TD) controls (ASD: monozygotic n = 19 pairs, dizygotic n = 29 pairs; TD: monozygotic n = 20 pairs, dizygotic n = 14 pairs). Pearson correlations that were significant at p < 0.05 (solid line) are displayed for twin pairs with ASD. These correlations were also significant across all individuals but not separately in TD control twins.
Discussion
Complex interactions between genetic and environmental factors during development may mediate some of the variability in neurobiological alterations and symptom presentation across individuals with ASD,21,25,26 such as the relationship between CSC and RRB.9,10 In the current study, we found substantial genetic influences on subcortical (i.e., striatum and thalamus) and frontal (i.e., ACC and OFC) CSC regions in both twins with ASD and TD controls, but cortical thickness of grey matter in the ACC and OFC was predominantly influenced by environmental factors in only twins with ASD. Differences in cortical grey matter of the OFC were also associated with the severity of RRB symptoms, as assessed by 2 independent measures, and comparisons across zygosity subgroups indicated that environmental factors most likely contributed to these brain–behaviour relationships. Potential environmental contributions to the relationship between CSC and RRB were further supported by the observation of differences in RRB severity in monozygotic twin pairs with ASD, who share a similar genetic background, as well as differences in grey matter thickness of the OFC in twin pairs discordant for ASD, who provide greater control for shared environmental influences. Cumulatively, our findings suggest that genetic and environmental factors differentially affect CSC structure in children with ASD, with genetic factors primarily contributing to subcortical development but environmental factors contributing to the development of frontal lobe grey matter to a larger extent in children with ASD compared with TD controls.
Previously, CSC have been implicated in ASD with reports of neurobiological alterations in this network compared with TD controls.11–20 Although reports vary across studies and developmental periods,47 CSC differences are relatively well supported across both basic science and clinical studies of ASD.9 Observations from the current study further support that there are neurobiological differences in CSC structures in children and adolescents with ASD and included indications of larger grey matter volume of the putamen, smaller grey matter volume of the thalamus and reduced cortical thickness of the OFC in twins with ASD compared with TD controls. However, these differences were only moderate and did not survive correction for multiple comparisons. Grey matter volume of the thalamus was also moderately reduced in twins with ASD compared with unaffected (discordant) co-twins, whereas cortical thickness of the OFC was moderately increased. Because co-twins provide greater control for potential confounding sources of variability, these findings suggest that differences in genetic and environmental contributions to the development of CSC regions may mediate some heterogeneity in ASD-related alterations across individuals. Thus, examining the influence of genetic and environmental factors on CSC regions in twins with ASD is an important step toward improving neurobiological models of this disorder.
The effects of genetic and environmental influences on brain development typically exhibit dynamic changes during different developmental periods. For instance, environmental contributions generally increase with age in evolutionary older structures, such as regions associated with basic sensory processing, but decrease with age in more recently evolved regions, such as prefrontal regions associated with higher-order processes such as executive control and language.24 Preliminary studies of twins with ASD suggest that this shift in genetic versus environmental control of brain development may be altered in children with ASD.21 Observations from the current study support these previous reports regarding genetic and environmental influences on global brain structure in twins with ASD and further indicate that subcortical CSC grey matter is primarily genetically mediated in children and adolescents with and without ASD, potentially to an even larger extent in individuals with ASD. Conversely, cortical CSC grey matter, particularly cortical thickness, may be primarily environmentally mediated in children and adolescents with ASD but not TD controls. Interestingly, investigations of young infants at high risk for ASD report that alterations in grey matter surface area are present very early during postnatal life, whereas alterations in cortical thickness may occur later during postnatal development.48 Thus, our findings suggest that the postnatal environment may have a larger impact on the development of the ACC and OFC in children with ASD, which could be a contributing factor to symptom presentation.
Neurobiological alterations in CSC regions, including the prefrontal cortex, are associated with RRB severity,15–20 suggesting that disruptions in CSC processing may mediate some of the extreme heterogeneity in symptom presentation across individuals with ASD. In the current investigation, we also found that twin pair differences in grey matter volume of the OFC were correlated with differences in the severity of autistic mannerisms, as measured by the SRS, as well as the stereotyped and compulsive behaviours, as measured by the RBS-R. These observations support the concept that structural variation in CSC regions, particularly the OFC,16 contribute to RRB severity in children and adolescents with ASD. Furthermore, these brain–behaviour relationships were very similar in monozygotic twin pairs, who share 100% of their genetic profiles, and dizygotic twin pairs, who differ by approximately 50%, which suggests that environmental factors may affect the relationship between grey matter development and RRB severity. The existence of differences in RRB severity in monozygotic twins concordant for ASD, as well as ACE modelling of RRB subdomains, further supports a relationship between environmental factors and RRB presentation. These clinical observations are also supported by animal models of RRB in which environmental deprivation can increase RRB expression, whereas environmental enrichment can reverse the phenotype.10 Overall, genetic factors may generally increase vulnerability for the development of ASD and contribute to the severity of stereotyped behaviours, potentially via pathways that affect cortical white matter, grey matter surface area21,48 and subcortical CSC regions, whereas environmental influences could play a role in the expression and severity of other RRB symptom domains through mechanisms that affect cerebellar white matter and cortical thickness.21,22 Additional research will be necessary to elucidate these complex brain–behaviour-environment interactions, but preliminary evidence from the current investigation suggests that the postnatal environment contributes to the relationship between CSC alterations and RRB presentation in children with ASD, which warrants further investigation.
Limitations
Several important limitations should be considered regarding the interpretation of our findings. First, our analyses applied a rather basic twin modelling approach because our sample was not large enough to apply more advanced twin modelling techniques, evident from the observation of nonsignificant or negative ICCs for several structural measures. Second, the examination of related individuals could have affected our general diagnostic group comparisons, but the evaluation of co-twins discordant for ASD also provided an especially powerful control group for potentially confounding sources of variability. Third, the use of 2 separate scanners could have introduced additional variability into our comparisons, although transformation of approximately one-third of the neuroimaging data was applied, and analyses based on a single scanner did not significantly differ from those for the full data set.21 Finally, our findings indicate the level of magnitude with which genetic and environmental factors may contribute to the development of CSC structure, as well as the relationship between CSC alterations and RRB severity, but not the specific mechanisms that contribute to these brain–behaviour relationships (e.g., genetic polymorphisms, DNA methylation levels, in utero drug exposure, perinatal complications or postnatal environmental exposures).
Conclusion
Brain structure is primarily genetically medicated during typical development, but there are also periods of increased sensitivity to environmental factors during different developmental periods. This is illustrated by the increased influence of environmental factors on lower-order sensory processing regions during postnatal development and diminished influence of environmental factors on higher-order frontal regions throughout the lifespan.24 Our findings indicate that these dynamic genetic and environmental interactions may be altered in children and adolescents with ASD, such that genetic contributions could have a greater impact on the development of subcortical grey matter, whereas environmental contributions could have a greater impact on the development of the frontal lobe, which may be susceptible to environmental influences for a longer period of time in children with ASD compared with TD controls. Environmental influences on the OFC may also mediate some heterogeneity in the presentation of RRB across individuals. Future investigations should assess the specific mechanisms that contribute to these complex interactions and identify the developmental periods and brain structures that may be particularly vulnerable to their effects. This will be particularly important for improving neurobiological modelling of ASD to increase the potential for neurobiological stratification in the future.
Acknowledgements
The authors thank the participants and their families, many of whom travelled great distances, for their involvement in this research.
Footnotes
Funding: This work was supported by a grant from the National Institute of Mental Health (R01MH083972 to A.Y.H.), and J. Hegarty was supported by the Bass Society Pediatric Fellowship Program. These funding sources had no involvement in this research, and the authors have no conflicts of interest to declare.
Competing interests: None declared.
Contributors: L. Lazzeroni, J. Hallmayer, A. Reiss and A. Hardan designed the study. J. Hegarty, M. Raman, S. Cleveland, O. Wolke, J. Phillips, A. Reiss and A. Hardan acquired the data, which J. Hegarty, L. Lazzeroni, M. Raman and A. Hardan analyzed. J. Hegarty and A. Hardan wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received February 12, 2019.
- Revision received April 2, 2019.
- Accepted June 10, 2019.