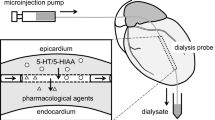
Summary
We have examined the local and systemic effects of clorgyline, tranylcypromine and deprenyl on extracellular serotonin (5-HT) and 5-hydroxyindoleacetic acid in the raphe nuclei and in frontal cortex of awake, freely-moving rats using microdialysis. When administered through the dialysis probe, monoamine oxidase (monoamine: oxygen oxidoreductase (deaminating), E.C. 1.4.3.4., MAO) inhibitors increased 5-HT output in a dose-dependent manner in both brain areas. The effects were more pronounced in the raphe nuclei for the three MAO inhibitors at all doses assayed.
When the monoamine oxidase inhibitors were given i.p., dialysate 5-HT increased dramatically, after tranylcypromine (15 mg/kg), in raphe nuclei and frontal cortex (area under the curve (AUC) to 4 h post-treatment: 63-fold and 11-fold, respectively) whereas the effects of clorgyline (10 mg/kg) were much less pronounced (+ 47% increase in the AUC for raphe nuclei, P < 0.09; + 18% increase in the AUC for frontal cortex, n.s.). Deprenyl (2.5 mg/kg, i.p.) induced a moderate (+ 22%) increase of dialysate 5-HT from the raphe nuclei but did not cause a change in dialysate 5-HT from the frontal cortex (+ 4%). However, clorgyline, or deprenyl, dramatically increased dialysate 5-HT in animals which had been pre-treated with the above dose of deprenyl, or clorgyline, respectively, showing that the blockade of both forms of MAO results in much larger increases of extracellular 5-HT than does the blockade of either form alone.
These results indicate that: (a) deamination by MAO participates actively in the control of the extracellular concentration of 5-HT in those areas of the brain that are rich in serotoninergic nerve terminals as well as in cell bodies, (b) in vivo, brain 5-HT is deaminated preferentially by MAO-A but its full inhibition does not result in an increased release of 5-HT, in spite of a large accumulation of 5-HT in the brain tissue, (c) MAO-B deaminates 5-HT when the A-form is inhibited (in this situation, MAO-B participates actively in the control of a releasable pool of 5-HT), (d) the raphe nuclei appears to be a preferential site of action of MAO inhibitors, administered either locally or systemically. These results may help to understand the model of action of MAO inhibitors as antidepressant drugs.
Similar content being viewed by others
References
Adell A, Sarna GS, Hutson PH, Curzon G (1989) An in vivo and behavioural study of the release of 5-HT by p-chloroamphetamine in reserpine-treated rats. Br J Pharmacol 97:206–212
Adell A, Artigas F (1991) Differential effects of clomipramine given locally or systemically on extracellular 5-hydroxytryptamine in raphe nuclei and frontal cortex. An in vivo microdialysis study. Naunyn-Schmiedeberg's Arch Pharmacol 343:237–244
Adell A, Carceller A, Artigas F (1991) Regional distribution of extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the brain of freely moving rats. J Neurochem 56:709–712
Adell A, Carceller A, Artigas F (1993) In vivo brain dialysis study of the somatodendritic release of serotonin in the raphe nuclei of the rat. Effects of 8-OH-DPAT. J Neurochem 60:1673–1681
Aghajanian GK, Sprouse J, Rasmussen K (1987) Physiology of the midbrain serotonin system. In: Meltzer HY (ed) Psychopharmacology: The Third Generation of Progress. Raven Press, New York, pp 141–149
Amrein R, Allen SR, Guentert TW, Hartmann D, Lorscheid T, Schoerlin MP, Vranesic D (1989) The pharmacology of reversible monoamine oxidase inhibitors. Br J Psychiatry 155:66–71
Artigas F, Adell A, Cortes R, Celada P (1991) Increased extracellular concentration of serotonin in the raphe nuclei after uptake inhibition measured with in vivo microdialysis. Secondary effects on 5-HT1A autoreceptors. Soc Neurosci Abstr 17:1488
Baker GB, Le Gatt DF, Coutts RT (1984) A comparison of the effects of acute and chronic administration of phenelzine and tranylcypromine on brain concentrations of 2-phenylethylamine, p-tyramine and tryptamine in the rat. In: Boulton AA, Baker GB, Dewhurst WG, Sandler M (eds) Neurobiology of the trace amines. Humana Press, Clifton (NJ), pp 277–282
Bel N, Artigas F (1992) Fluvoxamine increases preferentially extracellular 5-hydroxytryptamine in the raphe nuclei: an in vivo microdialysis study. Eur J Pharmacol 229:101–103
Blier P, De Montigny C (1985) Serotoninergic but not noradrenergic neurons in rat central nervous system adapt to long-term treatment with monoamine oxidase inhibitors. Neuroscience 16:949–955
Blier P, De Montigny C, Azzaro AJ (1986) Modification of the serotonergic and noradrenergic neurotransmissions by repeated administration of monoamine oxidase inhibitors: electrophysiological studies in the rat central nervous system. J Pharmacol Exp Ther 237: 987–994
Blier P, De Montigny C, Chaput Y (1987) Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J Clin Psychopharmacol 7:24S–35S
Bradberry CW, Sprouse JS, Sheldon PW, Aghajanian GK, Roth RH (1991) In vitro microdialysis: a novel technique for stimulated neurotransmitter release measurements. J Neurosci Methods 36:85–90
Cao Danh H, Strolin-Benedetti M, Dostert P (1984) Differential changes in monoamine oxidase A and B activity in aging rat tissues. In: Tipton KF, Dostert P, Strolin-Benedetti M (eds) Monoamine oxidase and disease. Academic Press, New York London, pp 301–317
Carboni E, Di Chiara G (1989) Serotonin release estimated by transcortical dialysis in freely-moving rats. Neuroscience 32:637–645
Celada P, Sarrias MJ, Artigas F (1990) Serotonin and 5-hydroxy-indoleacetic acid in plasma. Potential use as peripheral measures of MAO-A activity. J Neural Transm Suppl 32:149–154
Chen HY, Lin YP, Lee EHY (1992) Cholinergic and GABAergic mediations of the effects of apomorphine on serotonin neurons. Synapse 10:34–43
Crespi F, Garratt JC, Sleight AJ, Marsden CA (1990) In vivo evidence that 5-hydroxytryptamine (5-HT) neuronal firing and release are not necessarily correlated with 5-HT metabolism. Neuroscience 35:139–144
Fitzgerald LW, Kaplinsky L, Kimmelberg HK (1990) Serotonin metabolism by monoamine oxidase in rat primary astrocyte cultures. J Neurochem 55:2008–2014
Fowler CJ, Tipton KF (1982) Deamination of 5-hydroxytryptamine by both forms of monoamine oxidase in the rat brain. J Neurochem 38:733–736
Fueri C, Faudon M, Héry M, Héry F (1984) Release of serotonin from perikarya in cat nodose ganglia. Brain Res 304:173–177
Green AR, Youdim MBH (1975) Effects of monoamine oxidase inhibition by clorgyline, deprenyl or tranylcypromine on 5-hydroxytryptamine concentrations in rat brain and hyperactivity following subsequent tryptophan administration. Br J Pharmacol 55:415–422
Hall DWR, Logan BW, Parsons GH (1969) Further studies on the inhibition of monoamine oxidase by M&B 9302 (clorgyline) I substrate specificity in various mammalian species. Biochem Pharmacol 18:1447–1454
Hery F, Faudon M, Ternaux JP (1982) In vivo release of serotonin in two raphe nuclei (raphe dorsalis and magnus) of the cat. Brain Res Bull 8:123–129
Hutson PH, Sauna GS, O'Connell MT, Curzon G (1989) Hippocampal 5-HT synthesis and release in vivo is decreased by infusion of 8-OHDPAT into the nucleus raphe dorsalis. Neurosci Lett 100:276–280
Invernizzi R, Belli S, Samanin R (1992) Citalopram's ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug's effect in the frontal cortex. Brain Res 584:322–325
Kalén P, Strecker RE, Rosengren E, Bjorklund A (1988) Endogenous release of neuronal serotonin and 5-hydroxyindoleacetic acid in the caudate-putamen of the rat as revealed by intracerebral dialysis coupled to high-performance liquid chromatography with fluorimetric detection. J Neurochem 51:1422–1435
Kato T, Dong B, Ishii K, Kinemuchi H (1986) Brain dialysis: in vivo metabolism of dopamine and serotonin by monoamine oxidase A but not B in the striatum of unrestrained rats. J Neurochem 46: 1277–1282
Katz DM, Kimmelberg HK (1985) Kinetics and autoradiography of high affinity uptake of serotonin by primary astrocyte cultures. J Neurosci 5:1901–1908
Kimmelberg HK, Katz DM (1985) High-affinity uptake of serotonin into immunocytochemically identified astrocytes. Science 228: 889–891
Knoll J (1983) Deprenyl (selegiline): the history of its development and pharmacological action. Acta Neurol Scand Suppl 95:57–80
Lai JCK, Leung TKC, Guest JF, Lim L, Davison AN (1980) The monoamine oxidase inhibitors clorgyline and l-deprenyl also affect the uptake of dopamine, noradrenaline and serotonin by rat brain synaptosomal preparations. Biochem Pharmacol 29:2763–2767
Levitt P, Pintar JE, Breakefield XO (1982) Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA 79:6385–6389
Murphy DL, Aulakh CS, Garrick NA, Sunderland T (1987) Monoamine oxidase inhibitors as antidepressants: implications for the mechanism of action of antidepressants and the psychobiology of the affective disorders and some related disorders. In: Meltzer HY (ed) Psychopharmacology: The Third Generation of Progress. Raven Press, New York, pp 545–552
Neff NH, Yang HY, Fuentes JA (1974) The use of selective monoamine oxidase inhibitor drugs to modify amine metabolism in brain. In: Usdin E (ed) Neuropsychopharmacology of monoamines and their regulatory enzymes. Raven Press, New York, pp 49–57
O'Carroll AM, Fowler CJ, Phillips JP, Tobbia I, Tipton KF (1983) The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedeberg's Arch Pharmacol 322:198–202
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Sydney, Academic Press
Saura J, Kettler R, Da Prada M, Richards JG (1992) Quantitative enzyme radioautography with [3H]Ro 41–1049 and [3H]Ro 19–6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs and human brain. J Neurosci 12: 1977–1999
Sharp T, Zetterstrom T, Christmanson L, Ungerstedt U (1986) p-Chloroamphetamine releases both serotonin and dopamine into rat brain dialysates in vivo. Neurosci Lett 72:320–324
Sharp T, Bramwell SR, Grahame-Smith DG (1989) 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br J Pharmacol 96:283–290
Sleight AJ, Marsden CA, Martin KF, Palfreyman MG (1988) Relationship between extracellular 5-hydroxytryptamine and behaviour following monoamine oxidase inhibition and L-tryptophan. Br J Pharmacol 93:303–310
Sprouse JS, Aghajanian GK (1987) Electrophysiological responses of serotoninergic dorsal raphe neurons of 5-HT1A and 5-HT1B agonists. Synapse 1:3–9
Stenström A, Hardy J, Oreland L (1987) Intra- and extra-dopamine-synaptosomal localization of monoamine oxidase in striatal homogenates from four species. Biochem Pharmacol 36:2931–2935
The Parkinson study group (1989) Effect of deprenyl on the progression and disability in early Parkinson's disease. N Engl J Med 321:1364–1371
Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW (1985) Distinct monoamine oxidase A and B populations in primate brain. Science 230:181–183
Westlund KN, Denney RM, Rose RM, Abell CW (1988) Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience 25:439–456
Willoghby J, Glover V, Sandler M (1988) Histochemical localisation of monoamine oxidase A and B in rat brain. J Neural Transm 74:29–42
Wolf WA, Youdim MBH, Kuhn DM (1985) Does brain 5-HIAA indicate serotonin release or monoamine oxidase activity? Eur J Pharmacol 109:381–387
Yang HY, Neff NH (1973) β-Phenylethylamine: a specific substrate for type B monoamine oxidase of brain. J Pharmacol Exp Ther 187:365–371
Author information
Authors and Affiliations
Additional information
Correspondence to F. Artigas at the above address
Rights and permissions
About this article
Cite this article
Celada, P., Artigas, F. Monoamine oxidase inhibitors increase preferentially extracellular 5-hydroxytryptamine in the midbrain raphe nuclei. A brain microdialysis study in the awake rat. Naunyn-Schmiedeberg's Arch Pharmacol 347, 583–590 (1993). https://doi.org/10.1007/BF00166940
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00166940