Summary
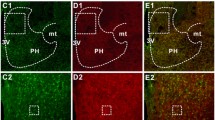
Corticotropin releasing factor (CRF), a neuropeptide synthesized in the parvocellular subnuclei of the hypothalamic paraventricular nucleus (PVN), takes part in the regulation of different stress evoked responses of the organism. In order to elucidate the role of the central adrenergic system in the regulation of these CRF-synthesizing neurons, a novel ultrastructural immunocytochemical dual localization technique was utilized. Phenylethanolamine-N-methyltransferase (PNMT), a specific enzyme marker for the central adrenaline system, and CRF-immunoreactive elements were simultaneously visualized in hypothalamic sections. PNMT-immunoreactive axon terminals established synaptic connections with somata, dendrites and spinous structures of CRF-producing neurons. This morphological finding indicates that the central adrenergic system directly influences CRF-synthesizing neurons in the PVN and provides basis for a more definitive pharmacological manipulation of this system.
Similar content being viewed by others
References
Agnati LF, Fuxe K, Yu Z-Y, Härfstrand A, Okret S, Wikström A-C, Goldstein M, Zoli M, Vale W, Gustafsson J-A (1985) Morphometrical analysis of the distribution of corticotrophin releasing factor, glucocorticoid receptor and phenylethanolamine-N-methyltransferase immunoreactive structures in the paraventricular hypothalamic nucleus of the rat. Neurosci Lett 54:147–152
Berod A, Hartman BK, Pujol JF (1981) Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of thyrosine hydroxylase. J Histochem Cytochem 29:844–850
Ganong WF (1980) Neurotransmitters and pituitary function: regulation of ACTH secretion. Fed Proc 39:2923–2930
Ganong WF, Kramer N, Salmon J, Reid IA, Lovinger R, Scapagnini U, Boryczka AT, Shackelford R (1976) Pharmacological evidence for inhibition of ACTH secretion by a central adrenergic system in the dog. Neuroscience 1:167–174
Hökfelt T, Fuxe K, goldstein M, Johansson O (1973) Evidence for adrenaline neurons in the rat brain. Acta Physiol Scand 89:286–288
Kalia M, Fuxe K, Goldstein M (1985a) Rat medulla oblongata. II. Dopaminergic, noradrenergic (A1 and A2) and adrenergic neurons, nerve fibers, and presumptive terminal processes. J Comp Neurol 233:308–332
Kalia M, Fuxe K, Goldstein M (1985b) Rat medulla oblongata. III. Adrenergic (C1 and C2) neurons, nerve fibers and presumptive terminal processes. J Comp Neurol 233:333–349
Labrie F, Giguere V, Proulx L, Lefevre G (1984) Interactions between CRF, epinephrine, vasopressin and glucocorticoids in the control of ACTH secretion. J Steroid Biochem 20:153–160
Leroux P, Pelletier G (1984) Radioautographic study of binding and internatlization of corticotropin-releasing factor by rat anterior pituitary corticotrophs. Endocrinology 114:14–21
Léránth C, Sakamoto H, MacLusky NJ, Shanabrough M, Naftolin F (1985) Application of avidin-ferritin and peroxidase as contrasting electron-dense markers for simultaneous electron microscopic immunocytochemical labelling of glutamic acid decarboxylase and tyrosine hydroxylase in the rat arcuate nucleus. Histochemistry 82:165–168
Liposits Zs, Görcs T, Sétáló GY, Lengvári I, Flerkó B, Vigh S, Schally AV (1983a) Ultrastructural characteristics of immunolabelled, corticotropin releasing factor (CRF)-synthesizing neurons in the rat brain. Cell Tissue Res 229:191–196
Liposits Zs, Görcs T, Török A, Domány S, Sétáló G (1983b) Simultaneou localization of two different tissue antigens based on the silver intensified PAP-DAB and on the traditional PAP-DAB method. Acta Morphol Hung 31:365–369
Liposits Zs, Lengvári I, Vigh S, Schally AV, Flerkó B (1983c) Immunohistological detection of degenerating CRF-immunoreactive nerve fibers in the median eminence after lesion of paraventricular nucleus of the rat. A light and electron microscopic study. Peptides 4:941–953
Liposits Zs, Sétáló G, Flerkó B (1984) Application of the silver-gold intensified 3,3′-diaminobenzidine chromogen to the light and electron microscopic detection of the LH-RH system of the rat brain. Neuroscience 13:513–525
Liposits Zs, Görcs T, Trombitás K (1985) Ultrastructural analysis of central serotoninergic neurons immunolabeled by silver-gold-intensified diaminobenzidine chromogen. J Histochem Cytochem 33:604–610
Liposits Zs, Phelix C, Paull WK (1986a) Electron microscopic analysis of tyrosine hydroxylase, dopamine-β-hydroxylase and phenylethanolamine-N-methyltransferase immunoreactive innervation of the hypothalamic paraventricular nucleus in the rat. Histochemistry (in press)
Liposits Zs, Sherman D, Phelix C, Paull WK (1986b) A combined light and electron microscopic immunocytochemical method for the simultaneous localization of multiple tissue antigens. Tyrosine hydroxylase immunoreactive innervation of corticotropin releasing factor synthesizing neurons in the paraventricular nucleus of the rat. Histochemistry (in press)
Merchenthaler I, Vigh S, Petrusz P, Schally AV (1982) Immunocytochemical localization of corticotropin releasing factor (CRF) in the rat brain. Am J Anat 165:385–396
Mezey É, Kiss JZ, Skirboll LR, Goldstein M, Axelrod J (1984) Increase of corticotropin-releasing factor staining in rat paraventricular nucleus neurons by depletion of hypothalamic adrenaline. Nature 310:140–141
Paull WK, Gibbs FP (1983) The corticotropin releasing factor (CRF) neurosecretory system in intact, adrenelectomized, and adrenalectomized-dexamethasone treated rats. Histochemistry 78:303–316
Rivier C, Vale W (1983) Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature 305:325–327
Saavedra JM, Palkovits M, Brownstein MJ, Axelrod J (1974) Localization of phenylethanolamine-N-methyltransferase in the rat brain nuclei. Nature 248:695–696
Sawchenko PE, Swanson LW (1981) Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science 214:685–687
Selye H (1937) Studies on adaptation. Endocrinology 21:169–188
Sternberger LA, Hardy PH Jr, Cuculis JJ, Meyer HG (1970) The unlabelled antibody enzyme method of immunochemistry. Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antiperoxidase) and its use in identification of spirochetes. J Histochem Cythochem 81:315–333
Swanson LW, Sawchenko PE, Berod A, Hartman BK, Helle KB, Van Orden DE (1981) An immunohistochemical study of the organization of catecholaminergic cells and terminal fields in the paraventricular and supraoptic nuclei of the hypothalamus. J Comp Neurol 196:271–285
Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186
Tilders FJH, Berkenbosch F, Smelik PG (1982) Adrenergic mechanisms involved in the control of pituitary-adrenal activity in the rat: a β-adrenergic stimulatory mechanism. Endocrinology 110:114–120
Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213:1394–1397
Vale W, Vaughan J, Smith M, Yamamoto G, Rivier J, Rivier C (1983) Effects of synthetic ovine corticotropin-releasing factor, glucocorticoids, catecholamines, neurohypophyseal peptides, and other substances on cultured corticotropic cells. Endocrinology 113:1121–1131
Van den Pol A (1985) Silver-intensified gold and peroxidase as dual ultrastructural immunolabels for pre- and postsynaptic neurotransmitters. Science 228:332–335
Wynn PC, Hauger RL, Holmes MA, Millan MA, Catt KJ, Aguilera G (1984) Brain and pituitary receptors for corticotropin releasing factor: localization and differential regulation after adrenalectomy. Peptides 5:1077–1084
Author information
Authors and Affiliations
Additional information
Supported by NIH grant NS19266
Rights and permissions
About this article
Cite this article
Liposits, Z., Phelix, C. & Paull, W.K. Adrenergic innervation of corticotropin releasing factor (CRF) — synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. Histochemistry 84, 201–205 (1986). https://doi.org/10.1007/BF00495783
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00495783