Abstract
Rationale
Acute depletion of brain tyrosine using a tyrosine-free amino acid mixture offers a nutritional approach to reduce central catecholamine function. Recent preclinical data suggest that tyrosine-free amino acid mixtures may have region-specific effects through targeting dopamine neurones.
Objectives
Here we used fos immunocytochemistry to examine the neuroanatomical sites of action of a tyrosine-free amino acid mixture administered either alone or combined with amphetamine.
Methods
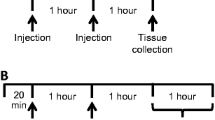
Rats (male, Sprague Dawley, 240–260 g) were administered (IP) either a tyrosine-free amino acid mixture (1 g/kg), or the same mixture supplemented with tyrosine and phenylalanine (1 g/kg). Mixtures were injected twice (1 h apart) followed 1 h later by amphetamine (2 mg/kg SC). Two hours later, cardiac perfusion was performed and brains were processed for fos immunocytochemistry. Fos positive cells were counted using computer imaging software.
Results
The tyrosine-free amino acid mixture alone did not alter fos expression in ten regions of the rat forebrain compared to saline controls. However, the mixture reduced the increase in fos expression evoked by amphetamine. This effect was region-specific and was greatest in caudate putamen, nucleus accumbens, bed nucleus stria terminalis and lateral habenula, and lacking in other areas including cingulate and insular cortices, lateral septum and central amygdaloid nucleus. Moreover, in most regions the effect of the tyrosine-free mixture was less after tyrosine and phenylalanine supplementation.
Conclusions
In summary, a tyrosine-free amino acid mixture reduced amphetamine-induced fos expression but this effect was region-specific and included dopamine-rich regions. These data further support the idea that tyrosine depletion strategies have potential as treatments for mania and other hyperdopaminergic states.





Similar content being viewed by others
References
Aronin N, Chase K, Sagar SM, Sharp FR, DiFiglia M (1991) N-Methyl-d-aspartate receptor activation in the neostriatum increases c-fos and fos-related antigens selectively in medium-sized neurons. Neuroscience 44:409–420
Biggio G, Porceddu M, Gessa GL (1976) Decrease of homovanillic, dihydroxyphenylacetic acid and cyclic-adenosine-3’,5’-monophosphate content by the acute administration of an amino acid mixture lacking tyrosine and phenylalanine. J Neurochem 26:1253–1255
Bing G, Stone EA, Zhang Y, Filer D (1992) Immunohistochemical studies of noradrenergic-induced expression of c-fos in the rat CNS. Brain Res 592:57–62
Boado R, Li J, Nagaya M, Zhang C, Pardridge W (1999) Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci USA 96:12079–12084
Day HE, Badiani A, Uslaner JM, Oates MM, Vittoz NM, Robinson TE, Watson SJ Jr, Akil H (2001) Environmental novelty differentially affects c-fos mRNA expression induced by amphetamine or cocaine in subregions of the bed nucleus of the stria terminalis and amygdala. J Neurosci 21:732–740
Dragunow M, Faull RL, Jansen KL (1990a) MK-801, an antagonist of NMDA receptors, inhibits injury-induced c-fos protein accumulation in rat brain. Neurosci Lett 109:128–133
Dragunow M, Robertson GS, Faull RL, Robertson HA, Jansen K (1990b) D2 dopamine receptor antagonists induce fos and related proteins in rat striatal neurons. Neuroscience 37:287–294
Fernstrom J (1983) Role of precursor availability in control of monoamine biosynthesis in brain. Physiol Rev 63:484–546
Fernstrom M, Fernstrom J (1995) Acute tyrosine depletion reduces tyrosine hydroxylation rate in rat central nervous system. Life Sci 57:PL97–PL102
Fu L, Beckstead RM (1992) Cortical stimulation induces fos expression in striatal neurons. Neuroscience 46:329–334
Graybiel A, Moratalla R, Robertson H (1990) Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA 87:6912–6916
Herrera D, Robertson H (1996) Activation of c-fos in the brain. Prog Neurobiol 50:83–107
Johansson B, Lindstrom K, Fredholm B (1994) Differences in the regional and cellular localisation of c-fos messenger RNA induced by amphetamine. Neuroscience 59:837–849
Kovacs K (1998) c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33:287–297
Lee Y, Hamamura T, Ohashi K, Miki M, Fujiwara Y, Kuroda S (2000) Carbamazepine suppresses methamphetamine-induced fos expression in a regionally specific manner in the rat brain. Possible neural substrates responsible for antimanic effects of mood stabilizers. Neuropsychopharmacology 22:530–537
McTavish SFB, Callado L, Cowen PJ, Sharp T (1999a) Comparison of the effects of alpha-methyl-p-tyrosine and a tyrosine-free amino acid load on extracellular noradrenaline in the rat hippocampus in vivo. J Psychopharmacol 13:379–384
McTavish SFB, Cowen PJ, Sharp T (1999b) Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology 141:182–188
McTavish SFB, McPherson M, Sharp T, Cowen PJ (1999c) Attenuation of some subjective effects of amphetamine following tyrosine depletion. J Psychopharmacol 13:144–147
McTavish SFB, McPherson MH, Harmer CJ, Clark L, Sharp T, Goodwin GM, Cowen PJ (2001a) Antidopaminergic effects of dietary tyrosine depletion in healthy subjects and patients with manic illness. Br J Psychiatry 179:356–360
McTavish SFB, Raumann B, Cowen PJ, Sharp T (2001b) Tyrosine depletion attenuates the behavioural stimulant effects of amphetamine and cocaine in rats. Eur J Pharmacol 424:115–119
Melamed E, Hefti F, Wurtman RJ (1980) Tyrosine administration increases striatal dopamine release in rats with partial nigrostriatal lesions. Proc Natl Acad Sci USA 77:4305–4309
Miller JC (1990) Induction of c-fos mRNA expression in rat striatum by neuroleptic drugs. J Neurochem 54:1453–1455
Nguyen T, Kosofsky B, Birnbaum R, Cohen B, Hyman S (1992) Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci USA 89:4270–4274
Paxinos G, Watson S (1986) The rat brain in stereotactic coordinates, 2nd edn. Academic Press, New York
Robertson HA, Peterson MR, Murphy K, Robertson GS (1989) D1-dopamine receptor agonists selectively activate striatal c-fos independent of rotational behaviour. Brain Res 503:346–349
Scally MC, Ulus I, Wurtman RJ (1977) Brain tyrosine level controls striatal dopamine synthesis in haloperidol-treated rats. J Neural Transm 41:1–6
Semba J, Sakai M, Miyoshi R, Mataga N, Fukamauchi F, Kito S (1996) Differential expression of c-fos mRNA in rat prefrontal cortex, striatum, N. accumbens and lateral septum after typical and atypical antipsychotics: an in situ hybridization study. Neurochem Int 29:435–442
Singewald N, Sharp T (2000) Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by fos immunocytochemistry. Neuroscience 98:759–770
Wurtman RJ, Hefti F, Melamed E (1980) Precursor control of neurotransmitter synthesis. Pharmacol Rev 32:315–335
Young ST, Porrino LJ, Iadarola MJ (1991) Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA 88:1291–1295
Acknowledgement
M. Le Masurier was supported by an MRC research studentship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Le Masurier, M., Cowen, P.J. & Sharp, T. Fos immunocytochemical studies on the neuroanatomical sites of action of acute tyrosine depletion in the rat brain. Psychopharmacology 171, 435–440 (2004). https://doi.org/10.1007/s00213-003-1607-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1607-7