Abstract
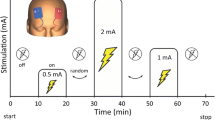
Transcranial magnetic stimulation (TMS) is increasingly utilized in clinical neurology and neuroscience. However, detailed knowledge of the impact and specificity of the effects of TMS on brain activity remains unresolved. We have used 14C-labeled deoxyglucose (14C-2DG) mapping during repetitive TMS (rTMS) of the posterior and inferior parietal cortex in anesthetized cats to study, with exquisite spatial resolution, the local and distant effects of rTMS on brain activity. High-frequency rTMS decreases metabolic activity at the primary site of stimulation with respect to homologue areas in the unstimulated hemisphere. In addition, rTMS induces specific distant effects on cortical and subcortical regions known to receive substantial efferent projections from the stimulated cortex. The magnitude of this distal impact is correlated with the strength of the anatomical projections. Thus, in the anesthetized animal, the impact of rTMS is upon a distributed network of structures connected to the primary site of application.








Similar content being viewed by others
References
Abramson BP, Chalupa LM (1985) The laminar distribution of cortical connections with the tecto- and cortico-recipient zones in the cat’s lateral posterior nucleus. Neuroscience 15:81–95
Ahmed B, Anderson JC, Douglas RJ, Martin KA, Nelson JC (1994) Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol 341:39–49
Albus K, Beckmann R (1980) Second and third visual areas of the cat: interindividual variability in retinotopic arrangement and cortical location J Physiol 299:247–276
Amassian VE, Quirk G J, Stewart M (1990) A comparision of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroen Clin Neuro 77:390–401
Araki S, Kawano A, Seldon HL, Shepherd RK, Funasaka S, Clark GM (2000) Effects of intracochlear factors on spiral ganglion cells and auditory brain stem response after long-term electrical stimulation in deafened kittens. Otolaryng Head Neck 122:425–433
Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm (2003) Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage 20:1685–96
Bohning DE, Pecheny AP, Epstein CM, Speer AM, Vincent DJ, Dannels W, George MS (1997). Mapping transcranial magnetic stimulation (TMS) fields in vivo with MRI. Neuroreport 8:2535–8
Buhl EH, Singer W (1989) The callosal projection in cat visual cortex as revealed by a combination of retrograde tracing and intracellular injection. Exp Brain Res 75:470–476
Civardi C, Cantello R, Asselman P, Rothwell JC (2001) Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage 14:1444–53
Einstein G (1996) Reciprocal projections of cat extrastriate cortex: I. Distribution and morphology of neurons projecting from posterior medial lateral suprasylvian sulcus to area 17. J Comp Neurol 376:518–529
Fierro B., Brighina F, Oliveri M., Piazza A, La Bua V, Buffa D, Bisiach E (2000) Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport 11:1519–21
Fox P, Ingham R, George MS, Mayberg HS, Ingham J, Roby J, Martin C, Jerabek P (1997) Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport 8:2787–2791
Galuske RA, Schmidt KE, Goebel R, Lomber SG, Payne BR (2002) The role of feedback in shaping neural representations in cat visual cortex. Proc Natl Acad Sci USA 99:17083–17088
George MS, Bellmaker RH (2000) Transcranial magnetic stimulation in neuropsychiatry. American Psychiatric, Washington DC
Gonzalez-Lima F (1992) Brain imaging of auditory functions in cats: studies with fluoroxyglucose autoradiography and cytochrome oxidase histochemistry. In: Gonzalez-Lima F, Finkenstaedt T, Scheich H (eds) Advances in metabolic mapping techniques for brain imaging of behavioral and learning functions. Kluwer, Dordrecht, pp 39–109
Grant S, Shipp S (1991) Visuotopic organization of the lateral suprasylvian area and of an adjacent area of the ectosylvian gyrus of cat cortex: a physiological and connectional study. Vis Neurosci 6:315–338
Harting J, Updyke B, Lieshout DP (1992) Corticotectal projections in the cat: anterograde transport studies of twenty-five cortical areas. J Comp Neurol 324:379–414
Hilgetag CC, Theoret H, Pascual-Leone A (2001) Enhanced visual spatial attention ipsilateral to rTMS-induced “virtual lesions” of human parietal cortex. Nature Neurosci 4:953–957
Kanaseki T, Sprague JM (1974) Anatomical organization of pretectal nuclei and tectal laminae in the cat. J Comp Neurol 158:319–337
Kobayashi M, Pascual-Leone A (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2:145–156
Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, Friston KJ (2003) Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci 23:5308–18
Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA (2001) Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiat 49:460–463
Lomber S, Payne BR (1996) Removal of two halves restores the whole—reversal of visual hemineglect during bilateral cortical or collicular inactivation in the cat. Vis Neurosci 1143–1156
Lowel L (2002) 2-deosyglucose architecture of the cat primary visual cortex. In: Payne BR, Peters A (eds) The cat primary visual cortex. Academic, San Diego, CA, pp 167–189
Luft AR, Kaelin-Lang A, Hauserc TK, Cohen LG, Thakorc NV, Hanley DF (2001) Transcranial magnetic stimulation in the rat. Exp Brain Res 140:112–21
Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M (2003) The anatomy of visual neglect. Brain 126:1986–97
Oliveri M, Rossini PM, Pasqualetti P, Traversa R, Cicinelli P, Palmieri MG, Tomaiuolo F, Caltagirone C (1999) Interhemispheric asymmetries in the perception of unimanual and bimanual cutaneous stimuli. A study using transcranial magnetic stimulation. Brain 122:1721–9
Oliveri M, Rossini PM, Cicinelli P, Traversa R, Pasqualetti P, Filippi MM, Caltagirone C (2000) Neurophysiological evaluation of tactile space perception deficits through transcranial magnetic stimulation. Brain Res Protoc 5:25–9
Oliveri M, Bisiach E, Brighina F, Piazza A, La Bua V, Buffa D, Fierro B (2001) rTMS of the unaffected hemisphere transiently reduces contralesional visuospatial hemineglect. Neurology 57:1338–1340
Palmer LA, Rosenquist AC, Tusa RJ (1978) The retinotopic organization of lateral suprasylvian visual areas in the cat. J Comp Neurol 2:237–256
Pascual-Leone A, Gomez-Tortosa E, Grafman J, Always D, Nichelli P, Hallett M (1994) Induction of visual extinction by rapid-rate transcranial magnetic stimulation of parietal lobe. Neurology 3:494–8
Pascual-Leone A, Davey N, Wassermann EM, Rothwell J, Puri, BK (2001) Handbook of transcranial magnetic stimulation. Arnold, London
Paus T (1999) Imaging the brain before, during, and after transcranial magnetic stimulation. Neuropsychologia 37:219–224
Paus T, Jech R, Thompson CJ, Comeau R., Peters T, Evans AC (1997) Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci 3178–3184
Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC (1998) Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol 1102–1107
Paus T, Castro-Alamancos MA, Petrides M (2001) Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci 14:1405–1411
Payne BR (1990) Representation of the ipsilateral visual field in the transition zone between areas 17 and 18 of the cat’s cerebral cortex. Vis Neurosci 4:445–474
Payne BR (1994) Neuronal interactions in cat visual cortex mediated by the corpus callosum. Behav Brain Res 64:55–64
Payne BR, Lomber SG (1999) A method to assess the functional impact of cerebral connections on target populations of neurons. J Neurosci Meth 86:195–208
Payne BR, Lomber SG (2002) The use of cooling deactivation to reveal the neural bases of lesion-induced plasticity of the developing and mature cerebral cortex. In: Lomber SG, Galuske RAW (eds) Virtual lesions: examining cortical function with reversible deactivation. Oxford University Press, Oxford, pp 163–188
Payne BR, Lomber SG (2003) Quantitative analyses of principal and secondary compound parieto-occipital feedback pathways in cat. Exp Brain Res 152:420–433
Payne BR, Siwek DF, Lomber SG (1991) Complex transcallosal interactions in visual cortex. Vis Neurosci 6:283–289
Payne BR, Lomber SG, Geeraerts S, Van der Gucht E, Vandenbussche E (1996) Reversible visual hemineglect. Proc Natl Acad Sci USA 93:290–4
Payne BR, Lomber SG, Rushmore RJ, Pascual-Leone A (2003) Cancellation of visuo-parietal lesion-induced spatial neglect. Exp Brain Res 150:395–398
Peters A, Payne BR (1993) Numerical relationships between geniculo-cortical afferents and pyramidal cell modules in cat primary visual cortex. Cereb Cortex 3:69–78
Peters A, Payne BR, Budd J (1994) A numerical analysis of the geniculocortical input to striate cortex in the monkey. Cereb Cortex 4:215–229
Raczkowski D, Rosenquist AC (1983) Connections of the multiple visual cortical areas with the lateral posterior-pulvinar complex and adjacent thalamic nuclei in the cat. J Neurosci 3:1912–1942
Robertson EM, Theoret H, Pascual-Leone A (2003) Studies in cognition: the problems solved and created by transcranial magnetic stimulation. J Cognitive Neurosci 15:948–960
Roth BJ, Saypol JM, Hallett M, Cohen LG (1991) A theoretical calculation of the electric field induced in the cortex during magnetic stimulation. Electroen Clin Neuro 81:47–56
Scannell JW, Burns GA, Hilgetag CC, O’Neil MA, Young MP (1999) The connectional organization of the cortico-thalamic system of the cat. Cereb Cortex 9:277–299
Schoppmann A, Stryker MP (1981) Physiological evidence that the 2-deoxyglucose method reveals orientation columns in cat visual cortex. Nature 293:574–576
Schurmann M, Nikouline VV, Soljanlahti S, Ollikainen M, Basar E, Ilmoniemi RJ (2001) EEG responses to combined somatosensory and transcranial magnetic stimulation. Clin Neurophysiol 112:19–24
Shapiro HM, Greenberg JH, Reivich M, Ashmead G, Sokoloff L (1978) Local cerebral glucose uptake in awake and halothane-anesthetized primates. Anesthesiology 48:97–103
Siebner, HR, Willoch F, Peller M, Auer C, Boecker H, Conrad B, Bartenstein P (1998) Imaging brain activation induced by long trains of repetitive transcranial magnetic stimulation. Neuroreport 9:943–948
Siebner H, Peller M, Bartenstein P, Willoch F, Rossmeier C, Schwaiger M, Conrad B (2001) Activation of frontal premotor areas during suprathreshold transcranial magnetic stimulation of the left primary sensorimotor cortex: a glucose metabolic PET study. Hum Brain Mapp 12:157–167
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Strafella AP, Paus T, Barrett J, Dagher A (2001) Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21:RC157
Symonds L, Rosenquist AC (1984) Laminar origins of visual corticocortical connections in the cat. J Comp Neurol 229:39–47
Tommerdahl, M, Whitsel BL, Favorov, OV, Metz CB, O’Quinn BL (1999) Responses of contralateral SI and SII in cat to same-site cutaneous flutter versus vibration. J Neurophysiol 82:1982–1992
Toop J, Burke RE, Dum BP, O’Donovan MJ, Smith CB (1982) 2-deoxyglucose autoradiography of single motor units: labeling of individual acutely active muscle fibers. J Neurosci Meth 5:283–289
Tusa RJ, Rosenquist AC, Palmer LA (1981) Multiple cortical visual areas: Visual field topography in the cat. In: Woolsey CN (ed) Cortical sensory organization: multiple visual areas. Humana, Clifton, NJ, pp 1–31
Updyke BV (1981) Projections from visual areas of the middle suprasylvian sulcus onto the lateral posterior complex and adjacent thalamic nuclei in the cat. J Comp Neurol 201:477–506
Vanduffel W, Vandenbussche E, Singer W, Orban GA (1995) Metabolic mapping of visual areas in the behaving cat: a [14C]2-deoxyglucose study. J Comp Neurol 354:161–180
Vanduffel W, Payne BR, Lomber SG, Orban GA (1997) Functional impact of cerebral connections. Proc Natl Acad Sci USA 94:7617–7620
Vuilleumier P, Hester D, Assal G, Regli F (1996) Unilateral spatial neglect recovery after sequential strokes. Neurology 1:184–189
Walsh V, Pascual-Leone A (2003) Neurochronometrics of mind: TMS in cognitive science. MIT Press, Cambridge, MA
Weissman JD, Epstein CM, Davey KR (1992) Magnetic brain stimulation and brain size: relevance to animal studies. Electroen Clin Neuro 85:215–219
Acknowledgements
Sadly, since the initial submission of this manuscript, Prof. Bertram Payne passed away, and this paper is dedicated to his memory. The experimental work at Boston University School of Medicine was supported by NS32137 and NS47754 (BRP), and the personnel were supported by: “La Caixa” (Spain) and the Spanish Ministry of Science and Technology (AV-C); NS44624 (RJR); NSF (SGL); K24 RR018875, the National Alliance for Research in Schizophrenia and Depression and the Goldberg Fund (APL).
Author information
Authors and Affiliations
Corresponding author
Additional information
B.R. Payne has passed away since this paper was completed.
Rights and permissions
About this article
Cite this article
Valero-Cabré, A., Payne, B.R., Rushmore, J. et al. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Exp Brain Res 163, 1–12 (2005). https://doi.org/10.1007/s00221-004-2140-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2140-6