Abstract
Purpose
Serotonergic brain regions play a crucial role in the modulation of emotion, and serotonergic dysfunction may contribute to several neurological disorders. [123I]ADAM is a novel SPECT tracer which binds with high affinity to serotonin transporters (SERT). The objective of this study was to compare different methods for the quantification of tracer binding and to develop a simplified single-scan protocol for this tracer, as well as to investigate its potential for characterisation of the transporter occupancy versus plasma concentration curve of a selective serotonin re-uptake inhibitor (SSRI).
Methods
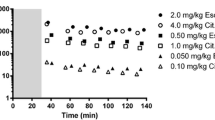
Dynamic SPECT scans were performed on 16 healthy volunteers after administration of ∼150 MBq [123I]ADAM. Data were acquired from the time of injection until ∼5.5 h after injection in 30- or 45-min sessions. Each subject was scanned twice: with and without pre-treatment with the SSRI citalopram in various dosage regimens. The plasma concentration of citalopram (C p) was determined from venous samples. Images were reconstructed by filtered back-projection with scatter and attenuation correction. Tracer binding was quantified for midbrain, striatum and thalamus using cerebellum as a reference region. Quantification was done by kinetic modelling, graphical analysis and multi-linear regression, as well as by the ratio method, with binding potential (BP2) as the outcome measure. The SERT occupancy by citalopram was determined relative to the baseline scan for each subject, and the occupancy versus C p curve was fitted with the E max model.
Results
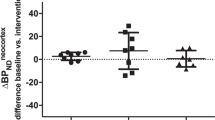
The highest binding of [123I]ADAM was in midbrain (mean baseline BP2±SD=1.31±0.29), with lower binding in thalamus (0.79±0.16) and striatum (0.66±0.13). There was good agreement between BP2 values obtained by different quantification methods. Using the ratio method, the best agreement with kinetic modelling was obtained with data from the time interval [200,260] min after injection. The fitting of the midbrain occupancy curve yielded a maximum occupancy of 84% and a plasma concentration required to reach 50% of the maximum of 2.5 ng/ml, with a goodness-of-fit variability of 13% (SD).
Conclusion
Binding of [123I]ADAM to SERT in midbrain can be quantified with a single scan starting 200 min after injection. However, the variability of estimated occupancy values may be too high for critical assessment of occupancy of SERT by SSRI.






Similar content being viewed by others
References
Owens MJ, Nemeroff CB. The serotonin transporter and depression. Depress Anxiety 1998;8 Suppl 1:5–12
Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology 1999;21 Suppl 2:99S–105S
Dolberg OT, Iancu I, Sasson Y, Zohar J. The pathogenesis and treatment of obsessive-compulsive disorder. Clin Neuropharmacology 1996;19:129–47
Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, et al. Reduced central serotonin transporters in alcoholism. Am J Psychiatry 1998;155:1544–9
Raisman R, Cash R, Agid Y. Parkinson’s disease: decreased density of 3H-imipramine and 3H-paroxetine binding sites in putamen. Neurology 1986;36:556–60
Palmer AM, Francis PT, Benton JS, Sims NR, Mann DM, Neary D, et al. Presynaptic serotonergic dysfunction in patients with Alzheimer’s disease. J Neurochemistry 1987;48:8–15
Tejani-Butt SM, Yang J, Pawlyk AC. Altered serotonin transporter sites in Alzheimer’s disease raphe and hippocampus. Neuroreport 1995;6:1207–10
Mann JJ. The role of in vivo neurotransmitter system imaging studies in understanding major depression. Biol Psychiatry 1998;44:1077–8
Oya S, Choi SR, Hou C, Mu M, Kung MP, Acton PD, et al. 2-((2-((dimethylamino)methyl)phenyl)thio)-5-iodophenylamine (ADAM): an improved serotonin transporter ligand. Nucl Med Biol 2000;27:249–54
Kauppinen TA, Bergström KA, Heikman P, Hiltunen J, Ahonen AK. Biodistribution and radiation dosimetry of [123I]ADAM in healthy human subjects: preliminary results. Eur J Nucl Med Mol Imaging 2003;30:132–6
Newberg AB, Plossl K, Mozley PD, Stubbs JB, Wintering N, Udeshi M, et al. Biodistribution and imaging with 123I-ADAM: a serotonin transporter imaging agent. J Nucl Med 2004;45:834–41
Huang WS, Ma KH, Cheng CY, Chen CY, Fu YK, Chou YH, et al. Imaging serotonin transporters with 123I-ADAM brain SPECT in healthy non-human primates. Nucl Med Commun 2004;25:515–9
Lin KJ, Yen TC, Wey SP, Hwang JJ, Ye XX, Tzen KY, et al. Characterization of the binding sites for 123I-ADAM and the relationship to the serotonin transporter in rat and mouse brains using quantitative autoradiography. J Nucl Med 2004;45:673–81
Asenbaum S, Füger B, Zettinig G, Dudczak R. 123-I ADAM and SPECT for investigating serotonin transporter in human. Nuklearmedizin 2003;6:A158
Catafau AM, Perez V, Penengo MM, Bullich S, De-Juan R, Puigdemont D, et al. Characterization and long-term test-retest reliability of the serotonin transporter SPECT ligand 123I-ADAM in healthy volunteers. J Nucl Med 2004;45 Suppl:260P
Ahonen AK, Kauppinen TA, Heikman P, Bergström KA, Launes J, Nikkinen P, et al. 123I labeled ADAM - A selective novel radioligand for imaging of serotonin transporters in the human brain. J Nucl Med 2002;43:232P
Acton PD, Choi SR, Hou C, Plossl K, Kung HF. Quantification of serotonin transporters in nonhuman primates using [123I]ADAM and SPECT. J Nucl Med. 2001;42:1556–62
Frokjaer VG, Pinborg LH, Madsen J, Knudsen GM. Evaluation of the serotonin transporter ligand [123I]ADAM for SPECT studies in humans. Neuroimage 2004;22 Suppl 2:T176
Erlandsson K, Sivananthan T, Lui D, Townsend CE, Lucas R, Ell PJ. Development of a simplified scanning protocol for the serotonin transporter SPET tracer [123I]ADAM. Nucl Med Commun 2004;25:405
Erlandsson K, Warrington S, Sivananthan T, Lui D, Spezzi A, Townsend CE, et al. Measuring SSRI occupancy of SERT using the novel SPET tracer [123I]ADAM. J Nucl Med 2004;45:397P
Erlandsson K, Sivananthan T, Lui D, Spezzi A, Townsend CE, Mu S, et al. Estimation of SSRI occupancy of SERT using the novel SPET tracer [123I]ADAM and simultaneous modeling. Neuroimage 2004;22 Suppl 2:T144–5
Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol 1997;340:249–58
Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 1997;283:1305–22
Lange K, Fessler JA. Globally convergent algorithms for maximum a posteri transmission tomography. IEEE Trans Im Proc 1995;4:1430–8
Ogawa K, Harat Y, Ichihara T, Kubo A, Hashimoto S. A practical method for position-dependent Compton-scatter correction in single emission CT. IEEE Trans Med Im 1991;10:408–12
Kak AC, Slaney M. Principles of computerized tomographic imaging. New York: IEEE Press; 1988
Chang LT. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci 1978;25:638–43
Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage 1996;4:153–8
Logan J, Fowler J, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990;10:740–7
Varga J, Szabo Z. Modified regression model for the Logan plot. J Cereb Blood Flow Metab 2002;22:240–4
Ichise M, Ballinger JR, Golan H, Vines D, Luong A, Tsai S, et al. Noninvasive quantification of dopamine D2 receptors with iodine-123-IBF SPECT. J Nucl Med 1996;37:513–20
Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol 1984;15:217–27
Kim KM, Watabe H, Onishi Y, Yonekura Y, Iida H. Effects of scatter correction on kinetic analysis in I-123 IBF SPECT study. Neuroimage 2002;16:S83
Fujita M, Varrone A, Kim KM, Watabe H, Zoghbi SS, Seneca N, et al. Effect of scatter correction on the compartmental measurement of striatal and extrastriatal dopamine D2 receptors using [123I]epidepride SPET. Eur J Nucl Med Mol Imaging 2004;31:644–54
Buchert R, Varga J, Mester J. Limitations of bi-linear regression analysis for the determination of receptor occupancy with positron emission tomography. Nucl Med Commun 2004;25:451–9
Dresel S, laFougere C, Makowski M, Meisenzahl E, Frodl T, Gildehaus F, et al. Imaging of the serotonin transporter in healthy controls: pharmacodynamics of [I-123]ADAM. J Nucl Med 2004;45 Suppl:261P
Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [11C]DASB PET imaging study. Am J Psychiatry 2001;158:1843–9
Asenbaum S, Fueger B, Zettinig G, Dudczak R. Serotonin transporter in human brain—application of 123I-ADAM, A new SPECT ligand. J Nucl Med 2003;44 Suppl:222P
Ahonen A, Koskela A, Kauppinen T, Puronto O, Nieminen-Wendt T, von Went L. Serotonin transporter availability in patients with Asperger syndrome; early results. Nuklearmedizin 2003;6:A158–9
Ahonen A, Heikman P, Kauppinen T, Koskela A, Bergström K. Serotonin transporter availability in drug free depression patients using a novel SERT ligand. Eur J Nucl Med Mol Imaging 2004;31 Suppl 2:S227–8
Koskela A, Kauppinen T, Keski-Rahkonen A, Sihvola E, Kaprio J, Bergström K, et al. Serotonin transporter availability in patients with bulimia nervosa using 123I-ADAM. Eur J Nucl Med 2004;31 Suppl 2:S228
Newberg A, Ploessl K, Wintering N, Alavi A, Udeshi M, Kung H. Age related changes in serotonin transporter binding measured by I-123 ADAM. J Nucl Med 2004;45 Suppl:279P
Mann JJ, Huang Y-Y, Arango V, Oquendo MA, Hastings R, Van Heertum RL, et al. A polymorphism of the serotonin transporter gene affects in vivo expression of transporter in human raphe nuclei as measured by positron emission tomography. Neuroimage 2004;22 Suppl 2:T47
Erlandsson K, Bressan RA, Mulligan RS, Ell PJ, Cunningham VJ, Pilowsky LS. Analysis of D2 dopamine receptor occupancy with quantitative SPET using the high affinity ligand [123I]epidepride: resolving conflicting findings. Neuroimage 2003;19:1205–14
Erlandsson K, Fujita M, Innis RB, Ell PJ, Pilowsky LS. The effect of lipophilic metabolites on reference tissue modelling of [123I]epidepride SPET data. J Nucl Med 2003;44:254P–5P
Acknowledgements
We want to thank the following for their invaluable help: At the INM, J. Bomanji, S. Gacinovic, S. Hughes, G. McNamara, N. Nagabhushan and R. Syed, and at HMR, S. Amin, S. Bakare, R. Dhadda, S. Eyers, A. Morgan, T. Nielsen, R. Ochiel, L. Stocking and P. Tsabedze. This study was conducted in accordance with applicable laws and regulations, good clinical practices, and the ethical principles that have their origin in the Declaration of Helsinki. The study was sponsored by Eli Lilly and Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erlandsson, K., Sivananthan, T., Lui, D. et al. Measuring SSRI occupancy of SERT using the novel tracer [123I]ADAM: a SPECT validation study. Eur J Nucl Med Mol Imaging 32, 1329–1336 (2005). https://doi.org/10.1007/s00259-005-1912-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1912-y