Abstract
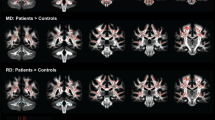
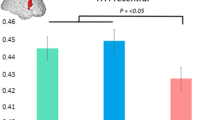
White matter (WM) degeneration is an important feature of Huntington’s disease (HD) neuropathology. To investigate WM degeneration we used Diffusion Tensor Imaging and Tract-Based Spatial Statistics to compare Fractional Anisotropy, Mean Diffusivity (MD), parallel diffusivity and perpendicular diffusivity (λ⊥) in WM throughout the whole brain in 17 clinically diagnosed HD patients and 16 matched controls. Significant WM diffusivity abnormalities were identified primarily in the corpus callosum (CC) and external/extreme capsules in HD patients compared to controls. Significant correlations were observed between motor symptoms and MD in the CC body, and between global cognitive impairment and λ⊥ in the CC genu. Probabilistic tractography from these regions revealed degeneration of functionally relevant interhemispheric WM tracts. Our findings suggest that WM degeneration within interhemispheric pathways plays an important role in the deterioration of cognitive and motor function in HD patients, and that improved understanding of WM pathology early in the disease is required.



Similar content being viewed by others
Abbreviations
- HD:
-
Huntington’s disease
- WM:
-
White matter
- DTI:
-
Diffusion Tensor Imaging
- TBSS:
-
Tract-Based Spatial Statistics
- FA:
-
Fractional Anisotropy
- λ⊥:
-
Perpendicular diffusivity
- λ||:
-
Parallel diffusivity
- MD:
-
Mean Diffusivity
- ROI:
-
Region-of-interest
- UHDRS:
-
Unified Huntington’s Disease Rating Scale
- MMSE:
-
Mini Mental State Examination
- BDI:
-
Beck Depression Inventory
- CAG:
-
Cytosine-adenine-guanine
- YSD:
-
Years since diagnosis
- NART:
-
National Adult Reading Test
- TR:
-
Repetition time
- TE:
-
Echo time
- FDT:
-
FMRIBs Diffusion Toolbox
References
Aylward, E. H., Anderson, N. B., Bylsma, F. W., Wagster, M. V., Barta, P. E., Sherr, M., et al. (1998). Frontal lobe volume in patients with Huntington’s disease. Neurology, 50(1), 252–8.
Bartzokis, G., & Tishler, T. A. (2000). MRI evaluation of basal ganglia ferritin iron and neurotoxicity in Alzheimer’s and Huntingon’s disease. Cellular and Molecular Biology (Noisy-le-grand), 46(4), 821–833.
Bartzokis, G., Cummings, J., Perlman, S., Hance, D. B., & Mintz, J. (1999). Increased basal ganglia iron levels in Huntington disease. Archives of Neurology, 56(5), 569–74.
Bartzokis, G., Lu, P. H., Tishler, T. A., Fong, S. M., Oluwadara, B., Finn, J. P., et al. (2007). Myelin breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochemical Research, 32(10), 1655–64.
Basser, P. J., & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B, 111(3), 209–19.
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–71.
Beglinger, L. J., Nopoulos, P. C., Jorge, R. E., Langbehn, D. R., Mikos, A. E., Moser, D. J., et al. (2005). White matter volume and cognitive dysfunction in early Huntington’s disease. Cognitive and Behavioral Neurology, 18(2), 102–7.
Behrens, T. E. J., Johansen-Berg, H., Woolrich, M. W., Smith, S. M., Wheeler-Kingshott, C. A. M., Boulby, P. A., et al. (2003). Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6(7), 750–7.
Blatter, D., Bigler, E., Gale, S., Johnson, S., Anderson, C., Burnett, B., et al. (1995). Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. American Journal of Neuroradiology, 16(2), 241.
Bohanna, I., Georgiou-Karistianis, N., Hannan, A. J., & Egan, G. F. (2008). Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington’s disease. Brain Research Reviews, 58(1), 209–25.
Ciarmiello, A., Cannella, M., Lastoria, S., Simonelli, M., Frati, L., Rubinsztein, D. C., et al. (2006). Brain White-Matter Volume Loss and Glucose Hypometabolism Precede the Clinical Symptoms of Huntington’s Disease, Journal of Nuclear Medicine (Vol. 47, pp. 215–222): Soc Nuclear Med.
Concha, L., Gross, D. W., Wheatley, B. M., & Beaulieu, C. (2006). Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage, 32(3), 1090–9.
de la Monte, S. M., Vonsattel, J. P., & Richardson, E. P., Jr. (1988). Morphometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington’s disease. Journal of Neuropathology and Experimental Neurology, 47(5), 516–25.
Fennema-Notestine, C., Archibald, S. L., Jacobson, M. W., Corey-Bloom, J., Paulsen, J. S., Peavy, G. M., et al. (2004). In vivo evidence of cerebellar atrophy and cerebral white matter loss in Huntington disease. Neurology, 63(6), 989–95.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–98.
Folstein, M. F., Robins, L. N., & Helzer, J. E. (1983). The mini-mental state examination. Archives of General Psychiatry, 40(7), 812.
Glenn, O. A., Henry, R. G., Berman, J. I., Chang, P. C., Miller, S. P., Vigneron, D. B., et al. (2003). DTI-based three-dimensional tractography detects differences in the pyramidal tracts of infants and children with congenital hemiparesis. Journal of Magnetic Resonance Imaging, 18(6), 641–8.
Group, H. S. (1996). Unified Huntington’s disease rating scale: reliability and consistency. Huntington study group. Movement Disorders, 11(2), 136–42.
Halliday, G. M., McRitchie, D. A., Macdonald, V., Double, K. L., Trent, R. J., & McCusker, E. (1998). Regional specificity of brain atrophy in Huntington’s disease. Experimental Neurology, 154(2), 663–72.
Jech, R., Klempir, J., Vymazal, J., Zidovska, J., Klempirova, O., Ruzicka, E., et al. (2007). Variation of selective gray and white matter atrophy in Huntington’s disease. Movement Disorders, 22(12), 1783–9.
Johansen-Berg, H., Della-Maggiore, V., Behrens, T. E. J., Smith, S. M., & Paus, T. (2007). Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage, 36, 16–21.
Kassubek, J., Bernhard Landwehrmeyer, G., Ecker, D., Juengling, F., Muche, R., Schuller, S., et al. (2004). Global cerebral atrophy in early stages of Huntington’s disease: quantitative MRI study. NeuroReport, 15(2), 363.
Kloppel, S., Draganski, B., Golding, C. V., Chu, C., Nagy, Z., Cook, P. A., et al. (2008). White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington’s disease. Brain, 131(Pt 1), 196–204.
Lee, W. C. M., Yoshihara, M., & Littleton, J. T. (2004). Cytoplasmic aggregates trap polyglutamine-containing proteins and block axonal transport in a Drosophila model of Huntington’s disease. Proceedings of the National Academy of Science, 101(9), 3224–9.
Li, H., Li, S. H., Cheng, A. L., Mangiarini, L., Bates, G. P., & Li, X. J. (1999). Ultrastructural localization and progressive formation of neuropil aggregates in Huntington’s disease transgenic mice. Human Molecular Genetics, 8(7), 1227–36.
Li, H., Li, S. H., Yu, Z. X., Shelbourne, P., & Li, X. J. (2001). Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. The Journal of Neuroscience, 21(21), 8473.
Mann, D. M., Oliver, R., & Snowden, J. S. (1993). The topographic distribution of brain atrophy in Huntington’s disease and progressive supranuclear palsy. Acta Neuropathologica, 85(5), 553–9.
Mascalchi, M., Lolli, F., Della Nave, R., Tessa, C., Petralli, R., Gavazzi, C., et al. (2004). Huntington disease: volumetric, diffusion-weighted, and magnetization transfer MR imaging of brain. Radiology, 232(3), 867–73.
Mori, S., & Wakana, S. (2005). MRI atlas of human white matter.
Nelson, H. E., & Willison, J. (1982). National adult reading test (NART): Test manual. Windsor: Nfer-Nelson.
Nichols, T. E., & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping, 15(1), 1–25.
Ota, M., Sato, N., Ohya, Y., Aoki, Y., Mizukami, K., Mori, T., et al. (2006). Relationship between diffusion tensor imaging and brain morphology in patients with myotonic dystrophy. Neuroscience Letters, 407(3), 234–9.
Paulsen, J. S., Magnotta, V. A., Mikos, A. E., Paulson, H. L., Penziner, E., Andreasen, N. C., et al. (2006). Brain structure in preclinical Huntington’s disease. Biological Psychiatry, 59(1), 57–63.
Paulsen, J. S., Langbehn, D. R., Stout, J. C., Aylward, E., Ross, C. A., Nance, M., et al. (2008). Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. Journal of Neurology, Neurosurgery and Psychiatry, 79(8), 874–80.
Pierpaoli, C., Barnett, A., Pajevic, S., Chen, R., Penix, L. R., Virta, A., et al. (2001). Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage, 13(6 Pt 1), 1174–85.
Reading, S. A., Yassa, M. A., Bakker, A., Dziorny, A. C., Gourley, L. M., Yallapragada, V., et al. (2005). Regional white matter change in pre-symptomatic Huntington’s disease: a diffusion tensor imaging study. Psychiatry Research, 140(1), 55–62.
Rosas, H. D., Koroshetz, W. J., Chen, Y. I., Skeuse, C., Vangel, M., Cudkowicz, M. E., et al. (2003). Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology, 60(10), 1615–20.
Rosas, H. D., Hevelone, N. D., Zaleta, A. K., Greve, D. N., Salat, D. H., & Fischl, B. (2005). Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology, 65(5), 745–7.
Rosas, H. D., Tuch, D. S., Hevelone, N. D., Zaleta, A. K., Vangel, M., Hersch, S. M., et al. (2006). Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Movement Disorders, 21(9), 1317–25.
Rosas, H. D., Salat, D. H., Lee, S. Y., Zaleta, A. K., Pappu, V., Fischl, B., et al. (2008). Cerebral cortex and the clinical expression of Huntington’s disease: complexity and heterogeneity. Brain, 131(Pt 4), 1057–68.
Rosas, H. D., Lee, S. Y., Bender, A. C., Zaleta, A. K., Vangel, M., Yu, P., et al. (2009). Altered white matter microstructure in the corpus callosum in Huntington’s disease: implications for cortical “disconnection”. Neuroimage, 49(4), 2995–3004.
Sapp, E., Penney, J., Young, A., Aronin, N., Vonsattel, J. P., & DiFiglia, M. (1999). Axonal transport of N-terminal huntingtin suggests early pathology of corticostriatal projections in Huntington disease. Journal of Neuropathology and Experimental Neurology, 58(2), 165–73.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), 1487–505.
Song, S. K., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage, 20(3), 1714–22.
Stoffers, D., Sheldon, S., Kuperman, J. M., Goldstein, J., Corey-Bloom, J., & Aron, A. R. (2010). Contrasting gray and white matter changes in preclinical Huntington disease: an MRI study. Neurology, 74(15), 1208–16.
Sullivan, E. V., Rohlfing, T., & Pfefferbaum, A. (2008). Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiology of Aging
Sun, S. W., Liang, H. F., Le, T. Q., Armstrong, R. C., Cross, A. H., & Song, S. K. (2006). Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage, 32(3), 1195–204.
Sydykova, D., Stahl, R., Dietrich, O., Ewers, M., Reiser, M. F., Schoenberg, S. O., et al. (2007). Fiber connections between the cerebral cortex and the corpus callosum in Alzheimer’s disease: a diffusion tensor imaging and voxel-based morphometry study. Cerebral Cortex, 17(10), 2276.
Tabrizi, S. J., Langbehn, D. R., Leavitt, B. R., Roos, R. A., Durr, A., Craufurd, D., et al. (2009). Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol.
Tai, Y. F., Pavese, N., Gerhard, A., Tabrizi, S. J., Barker, R. A., Brooks, D. J., et al. (2007a). Imaging microglial activation in Huntington’s disease. Brain Research Bulletin, 72(2–3), 148–51.
Tai, Y. F., Pavese, N., Gerhard, A., Tabrizi, S. J., Barker, R. A., Brooks, D. J., et al. (2007b). Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain, 130(7), 1759.
Thieben, M. J., Duggins, A. J., Good, C. D., Gomes, L., Mahant, N., Richards, F., et al. (2002). The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain, 125(8), 1815.
Wade, A., Jacobs, P., & Morton, A. J. (2008). Atrophy and degeneration in sciatic nerve of presymptomatic mice carrying the Huntington’s disease mutation. Brain Research, 1188, 61–8.
Weaver, K. E., Richards, T. L., Liang, O., Laurino, M. Y., Samii, A., & Aylward, E. H. (2009). Longitudinal diffusion tensor imaging in Huntington’s Disease. Experimental Neurology, 216(2), 525–9.
Acknowledgements
This study was funded by grant number 234247 from the National Health and Medical Research Council (Australia). We sincerely thank all participants for their time and effort. We also thank the Brain Research Institute (Austin Hospital) for the use of the 3T GE scanner. This study was carried out at School of Psychology, Psychiatry and Psychological Medicine, Monash University, Clayton, Victoria 3800, Australia. IB is supported by an Australian Rotary Health scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material
Supplementary Fig. 1
The ability of TBSS to identify the white matter tract skeleton in controls and both mild and moderate HD patients. The image clearly demonstrates the ability of the automated TBSS method to identify the major white matter pathways. (JPEG 128 kb)
Supplementary Fig. 2
Effect of using different thresholds on the group tract maps in HD patients, in (A) tracts from the ROI where MMSE and λ^ were correlated; and (B) tracts from the ROI where UHDRS and MD were correlated. Images show pathways present in between 5.9% (1/17) and 100% (17/17) of HD patients. A final threshold of 47% (8/17) was chosen to identify pathways that were common amongst individuals while still allowing the variability amongst individuals to be represented. (JPEG 3377 kb)
Rights and permissions
About this article
Cite this article
Bohanna, I., Georgiou-Karistianis, N., Sritharan, A. et al. Diffusion Tensor Imaging in Huntington’s disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging and Behavior 5, 171–180 (2011). https://doi.org/10.1007/s11682-011-9121-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-011-9121-8