Abstract
Objectives
The aims of this study were to provide a systematic review and meta-analysis of the effects of atypical antipsychotics in children and adolescents on weight gain (primary objective) and other metabolic parameters (secondary objective).
Methods
A systematic literature review and meta-analysis of double-blind, randomized, controlled trials were conducted. The data sources used were as follows: EMBASE, PubMed, BIOSIS, International Pharmaceutical Abstracts, The Cochrane database (Clinical Trials), Clinical Trials Government Registry, The metaRegister of Controlled Trials, WHO (World Health Organization) Clinical Trials Registry Platform, and PsycINFO®. Hand searching was also carried out by examining the reference lists of identified studies. Double-blind, randomized, controlled trials investigating the metabolic adverse effects (weight gain, lipid, glucose, and prolactin level abnormalities) associated with atypical antipsychotic use in children and adolescents aged ≤18 years were included, irrespective of whether the investigation of adverse effects was a primary or secondary endpoint.
Results
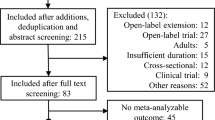
We identified 21 studies of drug versus placebo that met the inclusion criteria, with a total of 2,455 patients, 14 studies for risperidone (1,331 patients), three for olanzapine (276 patients), and four for aripiprazole (848 patients). Compared with placebo, the mean weight increases for each drug were olanzapine 3.45 kg (95 % CI 2.93–3.98), risperidone 1.77 kg (95 % CI 1.35–2.20), and aripiprazole 0.94 kg (95 % CI 0.65–1.24). Regarding other metabolic abnormalities, eight studies reported statistically significant increases in prolactin with risperidone; two reported a statistically significant increase in glucose, total cholesterol, and prolactin with olanzapine; and three studies reported a statistically significant decrease in prolactin with aripiprazole. Data on lipid, glucose, and prolactin level changes were too limited to allow us to perform a meta-analysis.
Conclusions
Olanzapine, risperidone, and aripiprazole were all associated with statistically significant weight gain. Olanzapine was associated with the most weight gain and aripiprazole the least. For the secondary outcome, although a number of active comparator trials were identified, data were not available for meta-analysis and were too limited to allow firm conclusions to be drawn.





Similar content being viewed by others
References
Jones P, Barnes TR, Davies L, et al. Randomised controlled trial of the effect of quality of life of second vs. first generation antipsychotic drugs in schizophrenia. Arch Gen Psychiatry. 2006;63:1079–87.
Malone RP, Sheikh R, Zito JM. Novel antipsychotic medications in treatment of children and adolescents. Psychiatr Serv. 1999;50:171–4.
Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behaviour disorders, and subaverage IQ. J Child Adolesc Psychopharmacol. 2004;14:243–54.
Glick I, Murray S, Hu R, et al. Treatment with atypical antipsychotics: new indications and new population. J Psychiatr Res. 2001;35:187–91.
Nasrallah N. A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology. 2003;28:83–96.
Findling RL, Robb A, Nyilas M, et al. A multiple-centre, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165:1432–41.
Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of antipsychotics in early onset schizophrenia and schizoaffective disorder. Am J Psychiatry. 2008;165:1420–31.
Chang KD. The use of atypical antipsychotics in pediatric bipolar disorder. J Clin Psychiatry. 2008;69(suppl.4):4–8.
Kryzhanovskaya LA, Plouch CK, Xu W, et al. The safety of olanzapine in adolescents with schizophrenia or bipolar disorder: a pooled analysis of 4 clinical trials. J Clin Psychiatry. 2009;70:247–58.
Correll CU. Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: a systemic review and pooled analysis of short term trials. J Am Acad Child Adolesc Psychiatry. 2007;46:687–700.
Wong ICK, Murray ML, Camilleri-Novak D, et al. Increased prescribing trends of paediatric psychotropic medications. Arch Dis Child. 2004;89:1131–2.
Rani F, Byrne PJ, Murray ML, Wong ICK, et al. Epidemiological features of antipsychotics prescribing to children and adolescents in primary care in United Kingdom. Pediatrics. 2008;121:1002–9.
Martin A, Leslie D. Trends in psychotropic medications costs for children and adolescents, 1997–2000. Arch Pediatr Adolesc Med. 2003;157:997–1004.
Burns MJ. The pharmacology and toxicology of atypical antipsychotic agents. Clin Toxicol. 2001;39:1–14.
Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006;51:480–91.
Kumra S, Oberstar JV, Sikich L, et al. Efficacy and tolerability of second generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull. 2008;34:60–71.
Fleischhaker C, Heister P, Hennighausen K, et al. Clinical drug monitoring in child and adolescent psychiatry: side effects of atypical neuroleptics. J Child Adolesc Psychopharmacol. 2006;16:308–16.
Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public health crisis common sense cure. Lancet. 2002;360:473–82.
Lissau I, Sorensen TI. Parental neglect during childhood and increased risk of obesity in young adulthood. Lancet. 1994;343:324–7.
Srinivasan SR, Myers L, Berenson G. Predictability of childhood adiposity and insulin for developing Insulin resistance syndrome in young adulthood. The Bogalusa Heart Study. Diabetes. 2002;51:204–9.
Johonson JG, Cohen P, Kasen S, et al. Childhood adversities associated with the risk for eating disorder or weight problems during adolescents or early adulthood. Am J Psychiatry. 2002;159:394–400.
Correll C, Carlson H. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–91.
Almandil NB, Wong ICK. Review on the current use of antipsychotic drugs in children and adolescents. Arch Dis Child Educ Pract Ed. 2011;96:192–6.
Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41.
Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;159:1686–96.
Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152–63.
Pringsheim T, Lam D, Ching H, Pattens S. Metabolic and neurological complications of second generation antipsychotic use in children: a systematic review and meta-analysis of randomised controlled trials. Drug Saf. 2011;34(8):651–68.
De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26:144–58.
Egger M, Smith GB, Altman D. Systemic review in health care: meta-analysis in context. London: BMJ Publishing Group; 2001. p. 23–66.
Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–900.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of randomised clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Review Manager (RevMan) [Computer program]. Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2008.
Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18:140–56.
Scahill L, Leckman JF, Schultz RT, et al. A placebo controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130–5.
Spettigue W, Buchholz A, Henderson K, et al. Evaluation of the efficacy and safety of olanzapine as an adjunctive treatment for anorexia nervosa in adolescent females: a randomized, double-blind, placebo-controlled trial. BMC Pediatr. 2008;8:4.
Delbello MP, Schwiers ML, Rosenberg HL, et al. A double blind randomised placebo controlled study of quetiapine as adjunctive treatment of adolescent mania. Psychiatry. 2002;41:1216–23.
Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:509–16.
Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, et al. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry. 2001;62:239–48.
Van Bellinghen M, De Troch C. Risperidone in the treatment of disturbances in children and adolescents with borderline intellectual functioning: a double-blind placebo controlled trial. J Child Adolesc Psychopharmacol. 2001;11:5–13.
Snyder R, Turgay A, Aman M, et al. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry. 2002;41:1026–36.
Aman MG, DeSmedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviours in children with subaverage intelligence. Am J Psychiatry. 2002;159:1337–46.
McCracken JT, McGough J, Shah B, etal.; Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–21.
Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114:e634–41.
Reyes M, Buitelaar J, Toren P, et al. A randomized, double-blind, placebo-controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. Am J Psychiatry. 2006;163:402–10.
Nagaraj R, Shinghi P, Malhi P. Risperidone in children with autism: randomised, placebo-controlled, double blind study. J Child Neurol. 2006;21(6):450–5.
Armenteros JL, Lewis JE, Davalos M. Risperidone augmentation for treatment-resistant aggression in attention-deficit/hyperactivity disorder: a placebo-controlled pilot study. J Am Acad Child Adolesc Psychiatry. 2007;46:558–65.
Anderson GM, Scahill L, McCracken JT, et al. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61:545–50.
Luby J, Mrakotsky C, Stalets MM, et al. Risperidone in preschool children with autistic spectrum disorders: An investigation of safety and efficacy. J Child Adolesc Psychopharmacol. 2006;16:575–87.
Haas M, Unis AS, Armenteros J, et al. A 6 week randomized double blind placebo controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol. 2009;19(6):611–21.
Haas M, Delbello MP, Pandina G, et al. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized double blind placebo controlled study. Bipolar Disord. 2009;11(11):687–700.
Hollander E, Wasserman S, Swanson EN, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16:541–8.
Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164:1547–56.
Kryzhanovskaya L, Schulz SC, McDougle C, et al. Olanzapine versus placebo in adolescents with schizophrenia: A 6 week randomised double blind placebo controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:60–70.
Tramontina S, Zeni CP, Ketzer CR, et al. Aripiprazole in children and adolescents with bipolar disorder co-morbid with attention-deficit/hyperactivity disorder: a pilot randomized clinical trial. J Clin Psychiatry. 2009;70:756–64.
Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124:1533–40.
Marcus RN, Owen R, Kamen L, et al. A placebo controlled fixed does study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1110–9.
Barzman DH, Delbello MP, Adler CM, et al. The efficacy and tolerability of quetiapine versus divalproex for the treatment of impulsive and reactive aggression in adolescents with co-occurring bipolar disorder and disruptive behavior disorders. J Child Adolesc Psychopharmacol. 2006;16:665–70.
Keefe R, Seidman LJ, Christensen BK, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;166:985–95.
Buchsbaum MS, Haznedar MM, Aronowitz J, et al. FDG-PET in never-previously medicated psychotic adolescents treated with olanzapine or haloperidol. Schizophr Res. 2007;94:293–305.
Emsley RA, Risperidone working group. Risperidone in the treatment of first episode psychotic patients: a double blind multicentre study. Schizophr Bull. 1999;25:721–9.
Emsley R, Rabinowitz J, Medori R, et al. Remission in early psychosis: rates, predictors and clinical and functional outcome correlates. Schizophr Res. 2007;89:129–39.
Facorro BC, Iglesias RP, Bonilla MR, et al. A practical clinical trial comparing haloperidol, risperidone and olanzapine for acute treatment of first episode nonaffective psychosis. J Clin Psychiatry. 2006;67:1511–21.
Kumra S, Kranzler H, Gerbino-Rosen G, et al. Clozapine versus “high-dose” olanzapine in refractory early-onset schizophrenia: an open-label extension study. J Child Adolesc Psychopharmacol. 2008;18:307–16.
Findling RL, Kusumakar V, Daneman D, et al. Prolactin levels during long risperidone treatment in children and adolescents. J Clin Psychiatry. 2003;64:1362–9.
Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry. 2008;69(suppl 4):26–36.
Butterfield MI, Becker ME, Connor KM, et al. Olanzapine in the treatment of post-traumatic stress disorder: a pilot study. Inter Clin Psychopharmacol. 2001;16:197–203.
Bogenschutz MP, Nurnberg HG. Olanzapine versus placebo in the treatment of borderline personality disorder. J Clin Psychiatry. 2004;65:104–9.
Beasley CM, Sutton VK, Hamilton SH, et al. A double blind randomized placebo controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol. 2003;23:582–94.
Ratzoni G, Gothelf D, Brand-Gothelf A, et al. Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. J Am Acad Child Adolesc Psychiatry. 2002;41:337–43.
Ross RG, Novins D, Farley GK, et al. A 1-year open label trial of olanzapine in school age children with schizophrenia. J Child Adolesc Psychopharmacol. 2003;13:301–9.
Haapasalo-Pesu KM, Saarijärvi S. Olanzapine induces remarkable weight gain in adolescent patients. Eur Child Adolesc Psychiatry. 2001;10:205–8.
National Institute for Health and Clinical Excellence (NICE). Mental health and behavioural disorders. London: NICE; 2006.
Komossa K, Rummel-Kluge C, Schmid F, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews 2009; (4): CD006569.
Mir A, Shivakumar K, Williamson RJ, et al. Change in sexual dysfunction on aripiprazole: a switching or add-on study. J Psychopharmacol. 2008;22(3):244–53.
Aitchison KJ, Mir A, Shivakumar K, et al. Costs and outcomes associated with an aripiprazole add-on or switching open-label study in psychosis. J Psychopharmacol. 2011;25(5):675–84. doi:10.1177/0269881109358198.
Acknowledgments
I.C.K.W., F.M.C.B., K.J.A., M.L.M., and N.B.A. conceived the idea of the study. All authors were involved in the study design. N.B.A. and Y.L. analyzed the data; N.B.A., F.M.C.B., M.L.M., and I.C.K.W. interpreted the data. All authors had full access to the study data and can take responsibility for the integrity of the data and the accuracy of the data analysis. All authors drafted, revised, and approved the final manuscript. I.C.K.W. and F.M.C.B. supervised the study.
Conflicts of interest and funding
F.M.C.B. has received lecture fees, consultancy fees, research grants, and equipment grants from, and has been sponsored to attend conferences by, various pharmaceutical companies. He was previously editor-in-chief of a journal sponsored by GlaxoSmithKline. He has been asked to organize conferences supported by an unrestricted educational grant from Janssen-Cilag, a company marketing risperidone. None of these monies have been paid directly to F.M.C.B.; all monies since 2001 have been paid to his NHS Trust. F.M.C.B. has recently been sponsored to attend international epilepsy conferences by Eisai. No monies are currently being received from pharmaceutical companies, or from any source other than his employer, the NHS in the UK. K.J.A. has been on the Advisory Board for the Bristol-Myers Squibb and Otsuka Pharmaceuticals Ltd, and in addition, has received consultancy fees including payment for lectures and educational presentations from the same company. She was previously a member of various advisory boards, receiving consultancy fees and honoraria, and has received research grants from various companies, including Lundbeck and GlaxoSmithKline. She currently holds an Alberta Centennial Addiction and Mental Health Research Chair, funded by the Government of Alberta. I.C.K.W. has received research funding and honoraria from various pharmaceutical companies, including Janssen-Cilag and Bristol-Myers Squibb (manufacturers of antipsychotic medicines). I.C.K.W. is currently receiving funding from the EU Commission to investigate the safety of risperidone in children. M.L.M. has received funding from pharmaceutical companies (Shire and Pfizer), but none of the funding is related to this study. The authors N.B.A. and Y.L. declare that they have no conflict of interest. N.B.A. is supported by a scholarship from the Ministry of Higher Education in the Kingdom of Saudi Arabia. No additional sources of funding were used to prepare this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almandil, N.B., Liu, Y., Murray, M.L. et al. Weight Gain and Other Metabolic Adverse Effects Associated with Atypical Antipsychotic Treatment of Children and Adolescents: A Systematic Review and Meta-analysis. Pediatr Drugs 15, 139–150 (2013). https://doi.org/10.1007/s40272-013-0016-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-013-0016-6