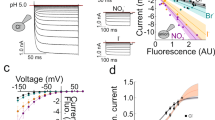
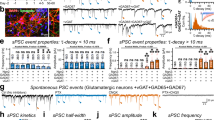
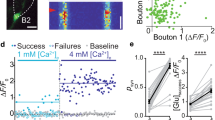
Abstract
Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system. Synaptic vesicles are loaded with neurotransmitter by means of specific vesicular transporters. Here we show that expression of BNPI, a vesicle-bound transporter associated with sodium-dependent phosphate transport1,2,3, results in glutamate uptake by intracellular vesicles. Substrate specificity and energy dependence are very similar to glutamate uptake by synaptic vesicles. Stimulation of exocytosis—fusion of the vesicles with the cell membrane and release of their contents—resulted in quantal release of glutamate from BNPI-expressing cells. Furthermore, we expressed BNPI in neurons containing GABA (γ-aminobutyric acid) and maintained them as cultures of single, isolated neurons that form synapses to themselves. After stimulation of these neurons, a component of the postsynaptic current is mediated by glutamate as it is blocked by a combination of the glutamate receptor antagonists, but is insensitive to a GABAA receptor antagonist. We conclude that BNPI functions as vesicular glutamate transporter and that expression of BNPI suffices to define a glutamatergic phenotype in neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ni, B., Rosteck, P. R. Jr., Nadi, N. S. & Paul, S. M. Cloning and expression of a cDNA encoding a brain-specific Na(+)-dependent inorganic phosphate cotransporter. Proc. Natl Acad. Sci. USA 91, 5607–5611 ( 1994).
Ni, B. et al. Molecular cloning, expression, and chromosomal localization of a human brain-specific Na+-dependent inorganic phosphate cotransporter. J. Neurochem. 66, 2227– 2238 (1996).
Bellocchio, E. E. et al. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J. Neurosci. 18, 8648– 8659 (1998).
Reimer, R. J., Fon, E. A. & Edwards, R. H. Vesicular neurotransmitter transport and the presynaptic regulation of quantal size. Curr. Opin. Neurobiol. 8, 405–412 (1998).
Hartinger, J., Stenius, K., Hogemann, D. & Jahn, R. 16-BAC/SDS-PAGE: a two-dimensional gel electrophoresis system suitable for the separation of integral membrane proteins. Anal. Biochem. 240, 126–133 (1996).
Dent, J. A., Davis, M. W. & Avery, L. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 16, 5867–5879 (1997).
Takamori, S., Riedel, D. & Jahn, R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J. Neurosci. 20, 4904–4911 ( 2000).
Parekh, D. et al. Characterization of a human pancreatic carcinoid in vitro : morphology, amine and peptide storage, and secretion. Pancreas 9, 83–90 (1994 ).
Naito, S. & Ueda, T. Adenosine triphosphate-dependent uptake of glutamate into protein I-associated synaptic vesicles. J. Biol. Chem. 258, 696–699 ( 1983).
Maycox, P. R., Hell, J. W. & Jahn, R. Amino acid neurotransmission: spotlight on synaptic vesicles. Trends Neurosci. 13, 83– 87 (1990).
Maycox, P. R., Deckwerth, T., Hell, J. W. & Jahn, R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J. Biol. Chem. 263 , 15423–15428 (1988).
Hell, J. W., Maycox, P. R. & Jahn, R. Energy dependence and functional reconstitution of the gamma-aminobutyric acid carrier from synaptic vesicles. J. Biol. Chem. 265, 2111–2117 ( 1990).
Stern-Bach, Y., Russo, S., Neuman, M. & Rosenmund, C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron 21, 907–918 ( 1998).
Raizen, D. M. & Avery, L. Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron 12, 483–495 (1994).
Lee, R. Y., Sawin, E. R., Chalfie, M., Horvitz, H. R. & Avery, L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J. Neurosci. 19, 159–167 (1999).
Aihara, Y. et al. Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J. Neurochem. 74, 2622–2625 (2000).
Ashery, U., Betz, A., Xu, T., Brose, N. & Rettig, J. An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur. J. Cell Biol. 78, 525–532 (1999).
Chen, C. & Okayama, H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7, 2745–2752 (1987).
Hell, J. W., Maycox, P. R., Stadler, H. & Jahn, R. Uptake of GABA by rat brain synaptic vesicles isolated by a new procedure. EMBO J. 7, 3023–3029 ( 1988).
Bekkers, J. M. & Stevens, C. F. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc. Natl Acad. Sci. USA 88, 7834–7838 (1991).
Rosenmund, C., Feltz, A. & Westbrook, G. L. Synaptic NMDA receptor channels have a low open probability. J. Neurosci. 15, 2788– 2795 (1995).
Hell, J. W. & Jahn, R. in Cell Biology: a Laboratory Handbook 1st edn (ed. Celis, J. E.) 567–574 (Academic, New York, 1994).
Lombard-Platet, G. & Jalinot, P. Funnel-well SDS-PAGE: a rapid technique for obtaining sufficient quantities of low-abundance proteins for internal sequence analysis. Biotechniques 15, 668–670, 672 (1993).
Acknowledgements
We thank J. Rettig for discussions and the help in the viral infection technique; D. Pommereit and Y. Stern-Bach for providing the non-desensitizing AMPA-receptor vectors GluR1L497Y and GluR2QL504Y–IRES–DsRed; M. Druminski, D. Diezmann, A. Bührmann, I. Herfort and N. Narajagan for their technical assistance; P. Holroyd for critical reading of this manuscript. We also thank The HHMI Biopolymer/W.M. Keck Foundation, Biotechnology Resource Laboratory at Yale University for amino-acid sequencing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takamori, S., Rhee, J., Rosenmund, C. et al. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 407, 189–194 (2000). https://doi.org/10.1038/35025070
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35025070
This article is cited by
-
Glutamate secretion by embryonic stem cells as an autocrine signal to promote proliferation
Scientific Reports (2023)
-
Oligodendrocyte death and myelin loss in the cuprizone model: an updated overview of the intrinsic and extrinsic causes of cuprizone demyelination
Molecular Neurodegeneration (2022)
-
Real time imaging of single extracellular vesicle pH regulation in a microfluidic cross-flow filtration platform
Communications Biology (2022)
-
VGLUT3 neurons in median raphe control the efficacy of spatial memory retrieval via ETV4 regulation of VGLUT3 transcription
Science China Life Sciences (2022)
-
DNA methylation of Vesicular Glutamate Transporters in the mesocorticolimbic brain following early-life stress and adult ethanol exposure—an explorative study
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.