Abstract
Gambling to recover losses is a common gaming behavior. In a clinical context, however, this phenomenon mediates the relationship between diminished control over gambling and the adverse socioeconomic consequences of gambling problems. Modeling loss-chasing through analogous behaviors in rats could facilitate its pharmacological investigation as a potential therapeutic target. Here, rats were trained to make operant responses that produced both food rewards, and unpredictably, imminent time-out periods in which rewards would be unavailable. At these decision points, rats were offered choices between waiting for these time-out periods to elapse before resuming responding for rewards (‘quit’ responses), or selecting risky options with a 0.5 probability of avoiding the time-outs altogether and a 0.5 probability of time-out periods twice as long as signaled originally (‘chase’ responses). Chasing behavior, and the latencies to chase or quit, during sequences of unfavorable outcomes were tested following systemic administration of the 5-HT1A receptor agonist, 8-OH-DPAT, the D2 receptor antagonist, eticlopride, and the D1 receptor antagonist, SCH23390. 8-OH-DPAT and eticlopride significantly reduced the proportion of chase responses, and the mean number of consecutive chase responses, in a dose-dependent manner. 8-OH-DPAT also increased latencies to chase. Increasing doses of eticlopride first speeded, then slowed, latencies to quit while SCH23390 had no significant effects on any measure. Research is needed to identify the precise cognitive mechanisms mediating these kinds of risky choices in rats. However, our data provide the first experimental demonstration that 5-HT1A and D2, but not D1, receptor activity influence a behavioral analog of loss-chasing in rats.
Similar content being viewed by others
INTRODUCTION
Gambling to recover losses is a prominent feature of recreational gambling and problem (or pathological) gambling (Dickerson et al, 1987). In a clinical context, excessive loss-chasing can involve persistent gambling to recover liabilities but diminishing resources to fund continued play (Lesieur, 1979). As such, this behavior represents an important mechanism that mediates between diminished control over gambling and the adverse family and socio-occupational consequences of clinically significant gambling problems (Lesieur, 1979). For these reasons, loss-chasing may represent a salient target for therapeutic intervention.
Despite the importance of loss-chasing to the clinical presentation of gambling problems, we know little about its neurochemical substrates. From a theoretical perspective, serotonin exerts complex influences on behavioral manifestations of impulsiveness (Winstanley et al, 2004), which both promotes loss-chasing (Breen and Zuckerman, 1999) and is exaggerated in samples of non-problem and problem gamblers (Blaszczynski et al, 1997). Serotonin activity also mediates learning from aversive events (Cools et al, 2008) and the ability to adjust behavior following punishing outcomes (Crockett et al, 2009). Intuitively, disturbances of these functions might promote loss-chasing behavior (Campbell-Meiklejohn et al, 2011).
Similarly, loss-chasing behavior is likely to involve dopamine functions that mediate the computation of action–value relationships (Schultz, 2010). Converging evidence implicates the activity of midbrain dopamine neurones, and its innervated forebrain sites, in the representation of risk and uncertainty (St Onge et al, 2011), and also in the internal framing of choice outcomes as involving gains or losses relative to some reference point (De Martino et al, 2006). However, while the above findings indicate that both serotonin and dopamine activity support the cognitive and affective processes likely to be involved in loss-chasing behavior, their role in this central feature of gambling has not been properly specified.
To fill this gap, we successfully developed a laboratory model of loss-chasing, which we have used to explore its neural and neurochemical substrates in humans (Campbell-Meiklejohn et al, 2008). Participants repeatedly choose between gambling to recover a loss of nominal value (at the risk of doubling its size) or quitting (and sustaining a certain loss). These dilemmas induce risk-seeking behavior in a variety of social and economic contexts (Kahneman and Tversky, 2000). In addition to showing that activity within the anterior cingulate cortex and subthalamic nucleus mediates loss-chasing choices (Campbell-Meiklejohn et al, 2008; Rogers et al, 2011), we have shown that tryptophan depletion reduces decisions to chase in healthy adults. By contrast, single treatments with 176 μg of the D2/D3 receptor agonist, pramipexole, increase the value of losses that participants are prepared to chase but reduce the value of losses surrendered when deciding to quit gambling (Campbell-Meiklejohn et al, 2011). Most recently, we have also demonstrated that single treatments with 20 mg of the psychostimulant, methylphenidate, attenuate the suppression of loss-chasing behavior observed as the magnitude of losses increases (Campbell-Meiklejohn et al, 2012).
These findings suggest that dopamine and serotonin have complementary roles in decisions to chase losses and/or decisions to quit gambling. However, research in humans is hampered by the limited pharmacological specificity of the available drug treatments, as well as the challenges associated with identifying their sites of action. Developing an animal model of loss-chasing behavior could broaden opportunities for pharmacological investigation. Here, we introduce a novel laboratory model of loss-chasing behavior in the rat. In the context of foraging behavior, animals tend to show risk aversion when choosing between actions associated with large unlikely rewards vs small likely rewards, but risk-seeking behavior when choosing between actions associated with short vs long delays to reward (Kacelnik and Bateson, 1996); in particular, animals will tolerate substantial risk to avoid longer intervals to the next opportunity to access reward (Kacelnik and Brito e Abreu, 1998). Hence, we trained rats to make simple operant responses that produced food rewards, and also, periodically and unpredictably, signaled imminent time-out periods in which reward would be unavailable. At these decision points, our animals were offered choices between waiting for the signaled time-out period to elapse before resuming responding for food rewards (‘quit’ responses), or selecting risky options with a 0.5 probability of avoiding the time-outs altogether and a 0.5 probability of time-out periods twice as long as signaled originally (‘chase’ responses). We report that, consistent with our observations with human subjects (Campbell-Meiklejohn et al, 2011), systemic administration of the 5-HT1A receptor agonist, 8-OH-DPAT, and the D2 receptor antagonist, eticlopride, diminishes the tendency to choose risky options to avoid time-outs. By contrast, administration of the D1 receptor antagonist, SCH23390, had no significant or marked effects on this analog of loss-chasing behavior.
MATERIALS AND METHODS
Subjects
Subjects were 24 male Long Evans rats, weighing 250–275 g at the start of testing. Behavioral testing and housing were in accordance with the Canadian Council of Animal Care and all experimental protocols were approved by the UBC Animal Care Committee.
Behavioral Apparatus
Testing took place in 8 standard 5-hole operant chambers (Med Associates, Vermont, CA, USA). The left wall of each chamber was concave, and contained an array of five response apertures, illuminated by light-emitting diodes. A food tray was located on the right wall. Responses in the apertures and food tray were monitored with infrared photocell beams. Sugar pellets (Noyes dustless pellets; Bioserv) were deposited into the tray from an external dispenser. The chamber was illuminated by a houselight. Auditory tones could be delivered into the chambers via a multiple tone generator.
Loss-Chasing Task
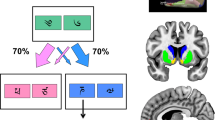
Full details of the training are described in the Supplementary Information, and a more detailed task schematic is provided as Supplementary Figure S1. The loss-chasing task involved two types of events: ‘partially reinforced’ (PR) trials and ‘chasing episodes’. On PR trials, rats were able to nose-poke at the food tray to start each trial, and to wait for hole 5 of the chamber to be illuminated before making single nose-poke responses there to earn single sugar pellets (Figure 1). Seventy percent of correct nose-pokes in hole 5 extinguished the light located inside hole 5, illuminated the tray light, and delivered one sugar pellet immediately. If rats responded in hole 5 before it was illuminated, the houselight was turned on for a time-out punishments of 5 s. These responses were scored as ‘premature responses’. If rats did not respond at hole 5 within 10 s of its illumination, the light in hole 5 was extinguished and the tray light illuminated again to signal that the rats should nose-poke there to start another trial. These trials were scored as ‘omitted responses’, but were not punished by time-outs.
Stimulus configurations for the choices in the loss-chasing task as implemented in 8 standard 5-hole operant chambers and performed following full training (see Supplementary Information). On ‘partially reinforced’ (PR) trials (70% of the total), nose-poke responses in hole 5 produced food rewards. During ‘chasing episodes’, the light in hole 5 flashed (0.5 Hz) and holes 1 and 3 were illuminated to indicate choice-points involving nose-poke responses in hole 3 that yielded fixed time-out periods of 4 s (‘quit’ responses) or nose-poke responses in hole 1 that yielded no time-out periods or time-out periods of 8 s with probabilities of 0.5 (1st ‘chase’). Following losing chase responses, the light in hole 1 flashed (0.5 Hz) and holes 2 and 3 were illuminated to indicate further choice-points involving nose-poke responses in hole 3 (‘quit’ responses) that yielded fixed time-outs of 8 s or nose-poke responses in hole 2 that yielded no time-out at all or time-out periods of 16 s, again with the probabilities of 0.5 (2nd ‘chase’). For 12 of the 24 rats, the 1st and 2nd chase responses were allocated to holes 1 and 2, as described above. For the remaining rats, the allocations were swapped so that the 1st and 2nd chase responses were allocated to hole 2 and hole 1, respectively. At every choice-point, the expected value in terms of the ‘opportunity cost’ of quit responses (4 or 8 s) and chase response (0.5 × 8 s and 0.5 × 16 s) were equal, indicating no actual time advantage for either behavioral strategy.
However, 30% of PR trials did not produce rewards following nose-pokes in hole 5. On these ‘chasing episodes’, the light in hole 5 began to flash at 0.5 Hz accompanied by a constant 4 kHz tone. Holes 1 and 3 were illuminated, signaling choice-points involving 1 of 2 further behaviors (Figure 1). Nose-poke responses at hole 3 were ‘quit’ responses. These responses extinguished the light in hole 1 and the flashing light in hole 5, but produced a flashing light in hole 3 that signaled a fixed 4 s time-out. At this time, the tone changed from 4 to 8 kHz. During time-outs, responses in any aperture had no programmed consequence; animals could neither terminate the time-out period nor earn reward. Once the 4 s time-out had elapsed, the light in hole 3 was extinguished and hole 5 was re-illuminated, accompanied by a 1 s 10 kHz tone. Now, rats could resume responding for reward at hole 5. Therefore, these quit responses were paired with fixed time-out penalties of 4 s during which reward was unavailable.
By contrast, nose-poke responses at hole 1 were ‘chase’ responses. These responses produced winning or losing outcomes with probabilities of 0.5. Winning outcomes canceled the time-out penalties, extinguished the lights in holes 1 and 3, re-illuminated the light in hole 5, and sounded a 1 s 10 kHz tone, allowing rats to resume responding for reward immediately.
Losing outcomes produced time-outs that lasted twice the length of those associated with quit responses, that is, 8 s. During these penalty periods, the light in hole 1 flashed at 0.5 Hz accompanied by a 12 kHz tone. At the end of this time-out, holes 2 and 3 were illuminated to signal 2nd choice-points involving further chase responses or quit responses (Figure 1). Nose-poke responses at hole 3 were again quit responses. These extinguished the flashing light in hole 1 and the light in hole 2, but produced a flashing light in hole 3, signaling the start of fixed 8 s time penalty. A tone of 8 kHz sounded throughout this time-out period. Once the time-out had elapsed, hole 5 was illuminated again, accompanied by a 1 s 10 kHz tone, allowing rats to resume responding for reward. Thus, quit responses at this 2nd choice-point of the chasing episode were paired with fixed time-out penalties of 8 s.
By contrast, nose-pokes at hole 2 were chase responses, again associated with winning or losing outcomes delivered with probabilities of 0.5. Following winning outcomes, the light in hole 2 was extinguished and the light in hole 5 was re-illuminated immediately, accompanied by a 1 s 10 kHz tone. This allowed rats to resume responding for reward immediately. However, following losing outcomes, the light in hole 2 flashed to signal a fixed 16 s time penalty, accompanied by a constant tone of 15 kHz sounded for the duration of this entire time-out period. Once this time-out had elapsed, the light in hole 5 was re-illuminated, with a 1 s 10 kHz tone, enabling the rats to resume responding for reward.
To summarize, chasing episodes first delivered choice-points involving quit responses (leading to fixed time penalties of 4 s) or chase responses involving a 0.5 probability of no time penalties or fixed time penalties of 8 s. Losing outcomes of this first chase response were followed by further choice-points involving further quit responses (leading to time penalties of 8 s) or chase responses involving a 0.5 probability of no time penalties or time penalties of 16 s. At every choice-point, the expected value in terms of the ‘opportunity cost’ associated with quit responses (4 or 8 s) and chase responses (0.5 × 8 s and 0.5 × 16 s) were equal, indicating no time advantage for either behavioral strategy. Animals were trained daily for 74 sessions—each 30 min—until stable chase over quit response preferences of at least 0.6 were established averaged the final five sessions. For 12 of the 24 rats, 1st and 2nd chase responses were allocated to hole 1 and to hole 2. For the remaining rats, these allocations were swapped so that the 1st and 2nd chase responses were allocated to hole 2 and to hole 1, respectively.
Dependent measures
Chasing episodes generated four dependent measures: (i) overall proportion of chase responses, initially calculated for 1st and 2nd choice-point separately; (ii) mean number of consecutive chase responses in each chasing opportunity; (iii) mean latencies for chase responses (s); and (iv) mean latencies for quit responses (s). PR trials yielded four additional measures: (v) mean latencies (s) to respond at hole 5 when it was illuminated; (vi) total number of premature responses made before hole 5 was illuminated; (vii) number of omitted responses in which rats failed to respond at hole 5 in time to earn food rewards; and (viii) mean latencies (s) to collect food rewards in the food tray.
Pharmacological Challenges
Performance was assessed following four treatments: 8-OH-DPAT (the selective 5-HT1A agonist with some additional affinity for 5-HT7 receptors and inhibitory properties over serotonin reuptake (Pucadyil et al, 2005); saline, 0.1, 0.3, and 0.6 mg/kg), eticlopride (a D2 receptor antagonist with some affinity for D3 and D4 receptors (Seeman and Ulpian, 1988); saline, 0.01, 0.03, and 0.06 mg/kg), and SCH23390 (a selective D1 receptor antagonist with affinity for D5 receptors (Bourne, 2001); saline, 0.001, 0.003, and 0.01 mg/kg). Treatments were administered 10 min before testing according to digram-balanced Latin square designs (Cardinal and Aitken, 2006). Drug/saline test day were preceded by treatment-free baseline days and followed by single days on which animals were not tested. Animals were tested drug free for at least 1 week between each treatment to re-establish a stable behavioral baseline.
All drug doses were calculated as the salt and dissolved in 0.9% sterile saline. All drugs were purchased from Sigma-Aldrich Canada (Oakville, ON, Canada), prepared fresh daily, and administered via the intraperitoneal route in a volume of 1 mg/ml.
Statistics
Data analyses proceeded in three stages. First, we analyzed the proportion of chase over quit responses on the 1st and 2nd choice-points of all chasing episodes following each treatment in repeated-measures analyses of variance (ANOVA) with treatment (saline vs three doses) and choice-point (1st vs 2nd) as two within-subject factors. As there were no significant changes in the proportion of chase responses on the 1st compared to 2nd choice-points following 8-OH-DPAT, eticlopride, or SCH23390 (all Fs3,15<1.585, nonsignificant (NS)), we pooled these data in all subsequent tests. Second, we tested for differences in the proportion of chase responses following each drug using repeated-measures ANOVAs with treatment as a single within-subjects factor.
Finally, following our experiments in human subjects (Campbell-Meiklejohn et al, 2011, 2012; Rogers et al, 2011), we conducted a final set of tests using only chasing episodes that terminated in either quit responses on the 1st choice-point, quit responses on the 2nd choice-point, or the maximum of two successive chase responses; that is, we excluded all chasing episodes that ended with winning outcomes in which time-outs were avoided. In this way, our analyses tested rats’ chasing behavior during runs of losing outcomes without the random interposition of winning outcomes. The dependent measures for these tests were (i) the mean number of consecutive chase responses per chasing opportunity (range 0–2); (ii) the mean latencies for chase responses (s); and (iii) the mean latencies for quit responses (s). All significant tests involving latencies were significant for both means and medians.
As loss-chasing is a common gambling strategy in humans and we wished to test how serotonergic and dopaminergic agents influenced an analog of this behavior, we excluded six rats that failed to establish a stable preference for chase over quit responses of <0.6 averaged over the final five training sessions, or chase over quit preferences of at least 0.5 chase in the saline sessions of the three drug treatments. Missing values for the higher doses of 8-OH-DPAT and eticlopride were replaced using the calculated series mean; maximum number of 10 cells. In all cases, reanalyses of the data without replacement of missing values yielded the same pattern of statistical results.
Proportions of chase responses were arcsine-transformed, as is appropriate whenever the variance of a measure is proportional to its mean (Howell, 1987); however, the figures and tables show untransformed data. We used Wilks’ lambda F-tests to assess treatment effects since multivariate ANOVA offers better protection against multicolinearity involving single factors (eg, saline and three doses) (Howell, 1987). Post-hoc differences between saline and doses were tested with paired t-tests.
RESULTS
8-OH-DPAT
During chasing episodes, higher doses of 8-OH-DPAT produced significant reductions in the overall proportion of rats’ chase responses compared with saline (see Figure 2; F(3, 15)=5.876, p<0.01) as well as significant reductions in the mean number of consecutive chase responses per chasing episode (Figure 3; F(3, 15)=12.256, p<0.0001). Mean latencies for chase responses were also significantly increased (see Figure 4; F3,15=40.353, p<0.0001). However, mean latencies for quit responses were not reliably changed (Figure 5; F3,15=1.86).
Mean proportion of ‘chase’ against ‘quit’ responses made by 18 rats while performing the loss-chasing task following three drug treatments: the 5-HT1A receptor agonist 8-OH-DPAT (saline, 0.1, 0.3, and 0.6 mg/kg), the D2 receptor antagonist eticlopride (saline, 0.01, 0.03, and 0.06 mg/kg), and the D1 receptor antagonist SCH23390 (saline, 0.001, 0.003, and 0.01 mg/kg). Dashed line represents perfectly balanced chase and quit responding.
Mean number of consecutive ‘chase’ responses per chasing episode in 18 rats while performing the loss-chasing task following three drug treatments: the 5-HT1A receptor agonist 8-OH-DPAT (saline, 0.1, 0.3, and 0.6 mg/kg), the D2 receptor antagonist eticlopride (saline, 0.01, 0.03, and 0.06 mg/kg), and the D1 receptor antagonist SCH23390 (saline, 0.001, 0.003, and 0.01 mg/kg).
Mean latencies for ‘chase’ responses made by 18 rats while performing the loss-chasing task following three drug treatments: the 5-HT1A agonist 8-OH-DPAT (saline, 0.1, 0.3, and 0.6 mg/kg), the D2 receptor antagonist eticlopride (saline, 0.01, 0.03, and 0.06 mg/kg), and the D1 receptor antagonist SCH23390 (saline, 0.001, 0.003, and 0.01 mg/kg).
Mean latencies for ‘quit’ responses made by 18 rats while performing the loss-chasing task following three drug treatments: the 5-HT1A agonist 8-OH-DPAT (saline, 0.1, 0.3, and 0.6 mg/kg), the D2 receptor antagonist eticlopride (saline, 0.01, 0.03, and 0.06 mg/kg), and the D1 receptor antagonist SCH23390 (saline, 0.001, 0.003, and 0.01 mg/kg).
On the PR trials, 8-OH-DPAT altered several behavioral measures (see Supplementary Table S1). Higher doses tended to increase the rats’ mean latencies to make simple nose-poke responses for food rewards at hole 5 (F3,15=2.994, p=0.082). 8-OH-DPAT also significantly reduced the number of premature responses (F3,15=6.476, p=0.005) while significantly increasing omitted responses (F3,15=17.50, p<0.0001). Mean latencies to collect food rewards from the food tray were also reliably increased (F3,15=8.115, p=0.005).
The number of PR trials completed by rats was significantly reduced with the higher doses of 8-OH-DPAT (Supplementary Table S1; F3,15=49.763, p<0.0001), raising the possibility that changes in loss-chasing responses were an artifact of diminished activity. However, the numbers of PR trials at the penultimate dose of 0.3 mg/kg remained substantial: mean=102.83±15.19, and the bias to make chase over quit responses during chasing episodes remained reliably above chance (0.5) following saline and all three doses (Figure 3; t(17)s>2.76, p<0.05).
Reanalysis of the chasing episodes with just saline, 0.1, and 0.3 mg/kg of 8-OH-DPAT, but omitting the highest dose of 0.6 mg/kg, demonstrated trend reductions in the proportion of chase over quit responses compared with saline (F2,16=2.35, p=0.127) but confirmed significant reductions in the number of consecutive chase responses per episode (F3,15=5.12, p<0.05). Post-hoc tests confirmed that the numbers of consecutive chase responses following 0.1 and 0.3 mg/kg were both significantly diminished against saline (Figure 3; t(17)=3.245, p<0.01 and t(17)=4.126, p<0.005). Reanalysis, without the highest dose of 8-OH-DPAT, also confirmed significant increases in latencies for chase responses (F2,16=20.491, p<0.0001).
Eticlopride
Eticlopride significantly reduced the overall proportion of chase over quit responses (Figure 2; F(3, 15)=3.885, p<0.05) and diminished the number of consecutive chase responses per chasing episode (Figure 3; F(3, 15)=5.348, p=0.01). Although eticlopride prolonged the latencies for chase responses at the highest dose of 0.06 mg/kg, the main effect of treatment was not significant (Figure 4; F3,15=2.539, p=0.096). By contrast, eticlopride significantly lengthened latencies to make quit responses at the penultimate dose of 0.03 mg/kg (Figure 5; F3,15=5.762, p=0.008).
On PR trials, eticlopride had virtually no impact on the rats’ mean latencies to make nose-poke responses for food rewards at hole 5 (Supplementary Table S2; F3,15<1.0). However, eticlopride treatment reliably diminished the number of premature responses compared with saline (F3,15=12.665, p<0.0001), and increased the number of omitted responses (F3,15=10.06, p<0.0001). The mean latencies for rats to collect their earned food rewards from the food tray were also significantly increased (Supplementary Table S2; F3,15=5.105, p=0.012).
Although higher doses of eticlopride significantly reduced the numbers of PR trials completed (F3,15=32.816, p<0.0001), the numbers completed at the penultimate dose of 0.03 mg/kg remained adequate: mean=95.72±11.27 (see Supplementary Table S2). In addition, the decision bias to make chase responses over quit responses during chasing episodes remained significant after each of the saline and all three drug dosages (t(17)s>3.268, p<0.01).
Reanalysis of chasing episodes with just saline, 0.01, and 0.03 mg/kg of eticlopride, but omitting the 0.06 mg/kg dose, confirmed the reliability of dose-dependent reductions in the proportion of chase responses (F2,16=3.923, p<0.05) and in the number of consecutive chase responses (F3,15)=6.844, p<0.01). The proportion of chase responses was reduced against saline following 0.01 mg/kg of eticlopride; however, this effect was not significant (t(17)=1.75, p=0.098). The proportion of chase responses was significantly reduced following 0.03 mg/mg (t(17)=2.422, p<0.05). The mean number of consecutive chase responses per chasing episode was significantly reduced compared with saline treatment following 0.01 mg/kg of eticlopride (t(17)=2.197, p<0.05) and 0.03 mg/mg (t(17)=3.186, p<0.036).
Finally, reanalysis with saline and the two lowest doses of eticlopride confirmed significant increases in the latencies for quit responses (Figure 5; F2,16=5.030, p=0.02). These treatment showed a significant quadratic trend (Figure 5; F1,17=8.093, p=0.011), being slightly but not significantly faster following 0.01 mg/kg compared with saline (t(17)=0.474, p=0.641), but significantly slower following 0.03 mg/kg compared with 0.01 mg/kg (t(17)=−3.926, p=0.004).
SCH23390
During chasing episodes, SCH23390 did not produce any substantial or significant changes in the proportion of chase responses compared with saline (Figure 2; F<1) or the mean number of consecutive chase responses per chasing episode (Figure 3; F<1). Mean latencies to make chase responses were not significantly affected (Figure 4; F3,15=1.903, p=0.172) nor were mean latencies to make ‘quit’ responses (Figure 5; F3,15=1.31).
On PR trials, SCH23390 had virtually no impact on the rats’ mean latencies to make nose-poke responses for earned food rewards at hole 5 (Supplementary Table S3; F<1). Similarly, SCH23900 treatment had no reliable effects on the number of premature responses made compared with saline (F<1). However, it did tend to increase the number of omitted responses (Supplementary Table S3; F3,15=2.295, p=0.119) and also significantly increased latencies to collect earned food rewards from the magazine (F3,15=3.356, p=0.047).
Higher doses of SCH23390 tended to reduce the number of PR trials completed; however, this reduction was not quite significant (F3,15=2.762, p=0.078) and the mean number of trials completed following the penultimate dose of 0.003 mg/kg remained 148.22±3.52 (Supplementary Table S3). As above, the bias to chase rather than quit remained significant after saline and all SCH23390 dosages (t(17)s>9.767, p<0.0001). Reanalysis of the chasing episodes with just saline and the two lowest doses of 0.001 and 0.003 mg/kg failed to demonstrate any significant effects of SCH23390 on proportions of chase responses, number of consecutive chase responses, their latencies, or latencies of quit responses (all Fs<1).
DISCUSSION
Loss-chasing behavior consists of the repeated selection of risky gambles with the intention of recovering previous gaming losses, even at the risk of incurring still greater liabilities (Lesieur, 1979). The experiment reported here represents a preliminary attempt to build an analog model of this behavior in the rat to facilitate focused neuropsychological and pharmacological investigations. Reflecting what we know about risky behaviors in foraging contexts (Kacelnik and Brito e Abreu, 1998), our observations demonstrate that rats will take risks to avoid certain delays until the next opportunity to earn reward, even at the risk of incurring still longer delays. This behavior may be analogous to observations that, faced with certain losses of nominal reward, human subjects with relatively limited gambling participation will select risky options associated with no losses at all or losses of double their magnitude. In our previous experiments, we have observed that individuals will decide to chase on approximately 0.72±0.02 of opportunities offered (Campbell-Meiklejohn et al, 2008). Here, we observed even higher rates of chase responses in rats at approximately 0.87±0.01 under saline conditions, suggesting that the ‘opportunity costs’ for earning food rewards are at least as salient to food-deprived rats as the costs of losing monetary rewards are to human subjects.
We found that treatment with the 5-HT1A receptor agonist, 8-OH-DPAT, and the D2 receptor antagonist, eticlopride, produced dose-dependent (and linear) reductions in the proportion of chase responses compared with saline, and the mean number of consecutive chase responses per chasing episode, with the eticlopride also producing specific increases in the times needed to make quit responses. These findings are consistent with observations from our previous experiments in human subjects (Campbell-Meiklejohn et al, 2011). By contrast, administration of the D1 receptor antagonist, SCH23390, produced no significant changes in any dependent measures of our loss-chasing task. These data extend recent evidence that aspects of human gambling behaviors can be successfully modeled in rats and pigeons (Rivalan et al, 2009; Scarf et al, 2011; Winstanley et al, 2011; Zeeb et al, 2009). Before discussing the implications of our findings, we first consider aspects of our experiment that are relevant to interpretation.
First, while the risk-seeking biases observed in rats presented with dilemmas involving certain and uncertain delays-to-reward appear comparable to the loss-chasing biases in humans, there remains some uncertainty about the specific cognitive representations and choice mechanisms that generate these decisional biases across species (Marsh and Kacelnik, 2002). In humans, loss-chasing is most frequently explained in terms of convex relationships between nominal value and subjective value or ‘utility’, such that the decreases in utility associated with unsuccessful decisions to chase are proportionately smaller than the decreases in utility associated with sustaining certain smaller losses (Kahneman and Tversky, 2000). However, in non-human species, risk-seeking behavior in the context of delays-to-reward may involve the operation of other mechanisms including negative energy budgets at the time of choice as described, for example, by Risk Sensitivity Theories (Kacelnik and Bateson, 1997). Alternatively, such choices may reflect the specific way that choice outcomes are represented in order to generate risk-averse behaviors for those that animals prefer to be large (eg, food magnitude) but risk-seeking behaviors for outcomes that animals prefer to be small (eg, delays-to-reward), as described by Scalar Utility Theory (Marsh and Kacelnik, 2002).
According to the Scalar Utility Theory, risk-seeking choices to avoid longer delays-to-reward, as in our loss-chasing task, reflect the operation of Weber’s fraction such that the variability in time interval estimates increases with their duration, leading to the systematic underestimation of longer delays-to-reward that can enhance the attractiveness of risky options, such as chase responses in our loss-chasing task (Kacelnik and Bateson, 1996; Marsh and Kacelnik, 2002). These perspectives upon the risk-seeking choices of animals and humans are not mutually exclusive; they seek to explain different aspects of the same observable behavior (Kacelnik and Bateson, 1997). However, research will need to identify the specific cognitive (and control) mechanisms underlying decisions to chase as implemented here for the rat, and to understand how variation in chasing behavior in this model relates to other behavioral models including delay discounting or response inhibition (Winstanley et al, 2004).
Second, we acknowledge that both dopamine and serotonin activity can influence cognitive processing of time intervals including delays-to-reward (Ho et al, 2002). While administration of 8-OH-DPAT does not impair the discrimination between longer and shorter time intervals (Body et al, 2002; Chiang et al, 2000), it can alter the differentiation of time intervals when shifting between operant responses (Body et al, 2002; Chiang et al, 2000). This raises the possibility that 8-OH-DPAT diminished chasing behavior by disturbing our rats’ ability to use delays-to-reward to regulate the balance between chase and quit responding. However, while eticlopride blocks the underestimation of time intervals induced by the D2 receptor agonist, quinpirole, it has no effect on its own at doses comparable to those used here (Cheung et al, 2007). Therefore, this and our own data suggest that D2 receptor activity influences risk-seeking choices when attempting to avoid delays-to-reward independently of any effects upon timing-based behaviors.
Third, we found that six rats (out of our entire sample of 24) failed to establish stable patterns of chasing behavior in either the baseline or saline testing sessions, and were excluded from our analyses. Risk-seeking choices shown by human subjects in their loss-chasing behavior also tend to be stable within subject, but show some variability between subject (Campbell-Meiklejohn et al, 2008). Identifying the sources of variability in the choices of rats during performance of our loss-chasing task may help to identify biological predispositions to gambling behaviors in the same way that variability in impulse control functions and faster acquisition of drug self-administration has been linked to altered D2/D3 receptor expression in the rat striatum (Dalley et al, 2007). Dopamine release within the ventral striatum can also impair adaptive decision making in pathological gamblers but facilitate adaptive choices in healthy controls (Linnet et al, 2011), suggesting that variability in subcortical dopamine activity can both promote and protect against gambling problems in distinct populations. In this experiment with rats, the small numbers of ‘chasers’ and ‘quitters’, and lack of an independent measure of neurotransmitter release and receptor expression, made it impossible to draw meaningful conclusions about the chasing behavior of these subgroups following drug challenges. However, experiments involving larger sample sizes could be designed to probe the important issue of individual differences in the propensity to chase losses.
Fourth, we note that the higher doses of all three drugs significantly increased (or tended to increase) the number of omitted responses and significantly reduced the number of PR trials completed per session, raising the possibility that our observations are artifacts of diminished motor activity. However, this is unlikely as the absolute number of trials completed was only markedly diminished at the highest doses, but remained substantial even at penultimate doses: 106.83±15.34 for 0.3 mg/kg of 8-OH-DPAT; 94.72±11.28 for 0.03 mg/kg of eticlopride; and 148.22±3.54 for 0.003 mg/kg of SCH23390. Crucially, the proportion of chase over quit responses remained significantly above chance following saline and all drug doses. This indicates that, while higher dosages reduced the number of PR trials completed (and, by implication, the number of chasing opportunities offered), these treatments did not abolish the consistent decision bias to make take risks to avoid delays-to-reward. Finally, repeating our statistical tests with just saline and the two lowest doses of 8-OH-DPAT and eticlopride, but omitting the highest doses, confirmed the statistical reliability of the altered chasing behavior seen here, and demonstrate its independence of overall behavioral activity.
Finally, we acknowledge that 8-OH-DPAT and eticlopride influenced rats’ behavior while responding for rewards on the PR trials of our loss-chasing task in ways that are both similar and different to previous investigations. Our loss-chasing task was modeled upon the operant characteristics of the 5-choice serial reaction time task (5-CSRTT) (Bari et al, 2008), providing comparable auxiliary measures of visuomotor performance. On the one hand, both 8-OH-DPAT and eticlopride tended to slow rats’ nose-poke responses following the locations of imperative visual signals at hole 5 to earn food reward on PR trials. Neither effect was statistically reliable; however, these effects are qualitatively similar to demonstrations that systemic administration of both compounds produce dose-dependent increases in the time needed to discriminate the spatial locations of visual targets in the 5-CSRTT (Carli and Samanin, 2000; van Gaalen et al, 2006). Similarly, although administration of eticlopride by itself does not influence discriminative accuracy in the 5-CSRTT (van Gaalen et al, 2006), it can slow latencies in simple reaction time tasks (Courtiere et al, 2003; Mayfield et al, 1993).
On the other hand, we found rather unexpected effects in relation to the control of premature responding on the PR trials, as an index of inhibitory control (Bari et al, 2008). Previously, systemic 8-OH-DPAT treatment has been shown to increase rates of premature responding in the 5-CSRTT (Carli and Samanin, 2000). In this experiment, the same treatments (and doses) significantly reduced premature responses, consistent with similar observations in an analog model of the Iowa Gambling Task for rats (Zeeb et al, 2009). Similarly, previous experiments found that eticlopride did not, on its own, influence the number of premature responses during performance of the 5-CSRTT, while SCH23390 dose-dependently diminished these kinds of errors (van Gaalen et al, 2006). Here, eticlopride treatment reduced rates of premature responses, while SCH23390 had no impact on this measure.
The reasons for these inconsistencies are unclear. However, we speculate that they reflect differences between the cognitive and motor demands of the 5-CSRTT and PR trials of our loss-chasing task. The former involves monitoring multiple spatial locations for visual targets and responding quickly and accurately in their locations. By contrast, the PR trials of our loss-chasing task require rats to monitor a single location for visual targets and execute simple nose-poke responses to gain rewards. On this view, our findings can be reconciled with earlier reports by noting the greater involvement of D1 receptor activity in selective attentional aspects of the 5-CSRTT (including speedy and accurate responding), and involvement of D2 receptors in the control over single prepared responses in our loss-chasing task (Eagle et al, 2011). More specifically, our finding that 8-OH-DPAT and eticlopride reduced the proportion of chase responses (and the number of consecutive chases per episode) and premature responses (on PR trials) point to links between aspects of gambling behavior and inhibitory control, exemplified by reports in human subjects that the control of betting behavior can be facilitated by successful inhibition of unrelated motor acts (Verbruggen et al, 2012).
Notwithstanding the above considerations, our findings extend earlier evidence that both dopaminergic and serotonergic mechanisms have significant roles in decisions to take risks to avoid negative outcomes in human subjects. Tryptophan depletion reduced the tendency of human subjects to continue gambling in the context of a run of bad gambling outcomes (Campbell-Meiklejohn et al, 2011). By contrast, tryptophan depletion had no impact upon the value of losses chased or the value of losses surrendered through decisions to quit. These results suggest that central serotonin activity governs the persistence of loss-chasing but not the evaluation of outcome values encountered during a run of losing gambles (Campbell-Meiklejohn et al, 2011). Our present results indicate that, compared with saline, the proportion of chase responses was reduced at all doses of 8-OH-DPAT (0.1, 0.3, and 0.6 mg/kg) while the latencies for these responses were increased. This suggests that these changes in risky choice reflect actions at presynaptic 5-HT1A receptors to diminish the activity of the dorsal raphe nuclei, thereby diminishing central 5-HT release (Carli and Samanin, 2000; Hedlund et al, 2004), and strengthens our hypothesis that serotonin activity supports the availability of loss-chasing as an aversively motivated escape strategy.
Both clinical evidence and experimentation have also linked D2 receptor activity to gambling behaviors (Dagher and Robbins, 2009, Voon et al, 2010, Zack and Poulos, 2004, Zeeb et al, 2009). Administration of the D2 receptor antagonist, haloperidol, can enhance the reward value of gambling experiences in pathological gamblers (Zack and Poulos, 2007) and alter betting patterns while playing slot machines (Tremblay et al, 2010). Previously, we found that single doses of pramipexole increased the value of losses that human subjects were prepared to chase (Campbell-Meiklejohn et al, 2011) while single doses of methylphenidate blocked the tendency of larger losses to suppress chasing behavior (Campbell-Meiklejohn et al, 2012). This suggests that dopamine activity mediate the evaluation of losses in relation to decisions to keep playing during runs of losing outcomes.
Of course, our rat model of loss-chasing did not afford any measure of how rats judged the value of increasing delays to the next opportunities to earn reward when selecting between chase or quit responses. However, the observation that eticlopride diminished the proportion of chase responses strengthens the evidence that D2 receptor activity mediates risky choices to avoid aversive consequences in an analog model of loss-chasing, and is consistent with the proposal that stimulation and, possibly, overstimulation of these receptors can influence the expression of this central feature of human gambling behaviors (Rogers et al, 2011; Voon et al, 2010). Moreover, the finding that latencies for quit responses were reduced following 0.01 mg/kg eticlopride compared with saline, but then increased following doses of 0.03 mg/kg, may reflect first presynaptic autoreceptor D2 activity (and enhanced dopamine release) at the lowest dose but post-synaptic D2 receptor activity at the higher dose, consistent with the biphasic effects of dopaminergic agents upon locomotor activity (Geyer et al, 1987; Smee and Overstreet, 1977).
Although further research will be needed to elucidate the psychological mechanism through which D2 activity influences loss-chasing behavior, we suggest three candidate mechanisms. First, one salient feature of our model is its emphasis upon repeated decisions to chase and the phenomena by which one risky gambling decision can easily lead to another, facilitating loss-chasing as a behavior that might become repetitious or automatic in vulnerable individuals (Campbell-Meiklejohn et al, 2008). Other data indicate that loss-chasing is associated with heightened impulsivity in gamblers (Breen and Zuckerman, 1999). As such, quit responses may require the ability to interrupt (or inhibit) decisions to chase to allow the re-evaluation of continued gambling against sustaining current losses. Consistent with evidence that eticlopride can facilitate the inhibition of dopamine-dependent impulsive responding (van Gaalen et al, 2006), increasing doses may have induced shifts from impulsive chase responses towards quit responses. Eticlopride also reduced, then increased, latencies to quit as the dose increased, suggesting that D2 receptor activity influences the duration of decisional processes that mediate the cessation of loss-chasing behavior.
Alternatively, D2 receptor activity is also involved in the acquisition of revised stimulus-reinforcement linkages in the form of reversal learning (Jocham et al, 2009). Overstimulation of D1 and D2 receptors following treatment with dopamine agonists has also been linked to faulty learning from the good and the bad outcomes of prior decisions (Frank and O’Reilly, 2006), and may contribute to the gambling (and impulse control) problems observed in some patients with Parkinson’s disease (Voon et al, 2010). According to this view, the antagonist actions of eticlopride at (possibly) post-synaptic D2 receptors may have facilitated learning from unsuccessful decisions to chase, raising the response threshold for further chasing behavior. The absence of any effects upon loss-chasing following SCH23390 suggests that D1 receptor activity, although implicated in probabilistic choice (St Onge et al, 2011), makes only limited contributions to loss-chasing.
Finally, administration of 8-OH-DPAT and D1/D2 receptor antagonist, haloperidol, can improve rats’ rate of reinforcement under differential reinforcement of low rates of responding schedules, principally through response suppression (rather than systematic shifts in inter-response times) (Britton and Koob, 1989; Cheng and Liao, 2007). This raises the possibility that the reduction of chase responses following treatment with 8-OH-DPAT and eticlopride was mediated, in part, by either indiscriminate choices between chase and quit responses at higher doses or enhanced tolerance of the time-outs following the selection of quit responses.
In summary, our experiment represents a preliminary test of a laboratory model of loss-chasing behavior in the rat. Using dilemmas involving certain fixed delays to the next opportunity to earn reward vs risky prospects associated with no delay at all or delays of two times the duration, we have shown that rats exhibit the same pattern of risk-seeking choices as seen in human subjects who gamble to recover losses. Consistent with our earlier experiments with human subjects (Campbell-Meiklejohn et al, 2011), our data show that both 8-OH-DPAT and eticlopride reduced decisions to chase losses, while eticlopride selectively modulated latencies associated with decisions to quit. Administration of the D1 receptor antagonist, SCH23390, had no significant effects on any measure of loss-chasing behavior. Although further research is needed to establish the precise cognitive mechanisms that mediate these risky choices in rats, our data provide evidence that 5-HT1A and D2 receptors make complementary contributions to loss-chasing behavior in rats and, possibly, humans.
References
Bari A, Dalley JW, Robbins TW (2008). The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 3: 759–767.
Blaszczynski A, Steel Z, McConaghy N (1997). Impulsivity in pathological gambling: the antisocial impulsivist. Addiction 92: 75–87.
Body S, Chiang TJ, Mobini S, Ho MY, Bradshaw CM, Szabadi E (2002). Effect of 8-OH-DPAT on temporal discrimination following central 5-hydroxytryptamine depletion. Pharmacol Biochem Behav 71: 787–793.
Bourne JA (2001). SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS Drug Rev 7: 399–414.
Breen R-B, Zuckerman M (1999). ‘Chasing’ in gambling behavior: personality and cognitive determinants. Pers Indiv Differ 27: 1097–1111.
Britton KT, Koob GF (1989). Effects of corticotropin releasing factor, desipramine and haloperidol on a DRL schedule of reinforcement. Pharmacol Biochem Behav 32: 967–970.
Campbell-Meiklejohn D, Simonsen A, Scheel-Kruger J, Wohlert V, Gjerloff T, Frith CD et al (2012). In for a Penny, in for a Pound: methylphenidate reduces the inhibitory effect of high stakes on persistent risky choice. J Neurosci 32: 13032–13038.
Campbell-Meiklejohn D, Wakeley J, Herbert V, Cook J, Scollo P, Ray MK et al (2011). Serotonin and dopamine play complementary roles in gambling to recover losses. Neuropsychopharmacology 36: 402–410.
Campbell-Meiklejohn DK, Woolrich MW, Passingham RE, Rogers RD (2008). Knowing when to stop: the brain mechanisms of chasing losses. Biol Psychiatry 63: 293–300.
Cardinal RN, Aitken M (2006) ANOVA for the Behavioural Sciences Researcher. Lawrence Erlbaum Associates: London.
Carli M, Samanin R (2000). The 5-HT(1A) receptor agonist 8-OH-DPAT reduces rats’ accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT(1A) receptors. Psychopharmacology (Berl) 149: 259–268.
Cheng RK, Liao RM (2007). Dopamine receptor antagonists reverse amphetamine-induced behavioral alteration on a differential reinforcement for low-rate (DRL) operant task in the rat. Chin J Physiol 50: 77–88.
Cheung TH, Bezzina G, Hampson CL, Body S, Fone KC, Bradshaw CM et al (2007). Effect of quinpirole on timing behaviour in the free-operant psychophysical procedure: evidence for the involvement of D2 dopamine receptors. Psychopharmacology (Berl) 193: 423–436.
Chiang TJ, Al-Ruwaitea AS, Mobini S, Ho MY, Bradshaw CM, Szabadi E (2000). Effects of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on performance on two operant timing schedules. Psychopharmacology (Berl) 151: 379–391.
Cools R, Robinson OJ, Sahakian B (2008). Acute tryptophan depletion in healthy volunteers enhances punishment prediction but does not affect reward prediction. Neuropsychopharmacology 33: 2291–2299.
Courtiere A, Hardouin J, Goujon A, Vidal F, Hasbroucq T (2003). Selective effects of low-dose dopamine D1 and D2 receptor antagonists on rat information processing. Behav Pharmacol 14: 589–598.
Crockett MJ, Clark L, Robbins TW (2009). Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J Neurosci 29: 11993–11999.
Dagher A, Robbins TW (2009). Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron 61: 502–510.
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K et al (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315: 1267–1270.
De Martino B, Kumaran D, Seymour B, Dolan RJ (2006). Frames, biases, and rational decision-making in the human brain. Science 313: 684–687.
Dickerson M, Hinchy J, Fabre J (1987). Chasing, arousal and sensation seeking in off-course gamblers. Br J Addict 82: 673–680.
Eagle DM, Wong JC, Allan ME, Mar AC, Theobald DE, Robbins TW (2011). Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. J Neurosci 31: 7349–7356.
Frank MJ, O’Reilly RC (2006). A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci 120: 497–517.
Geyer MA, Russo PV, Segal DS, Kuczenski R (1987). Effects of apomorphine and amphetamine on patterns of locomotor and investigatory behavior in rats. Pharmacol Biochem Behav 28: 393–399.
Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P (2004). 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol 487: 125–132.
Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E (2002). 5-Hydroxytryptamine and interval timing behaviour. Pharmacol Biochem Behav 71: 773–785.
Howell DC (1987) Statistical Methods for Psychology 2nd edn. Duxbury Press: Boston.
Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M (2009). Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J Neurosci 29: 3695–3704.
Kacelnik A, Bateson M (1996). Risky theories—the effects of variance on foraging decisions. Am Zool 36: 402–434.
Kacelnik A, Bateson M (1997). Risk-sensitivity: crossroads for theories of decision-making. Trends Cogn Sci 1: 304–309.
Kacelnik A, Brito e, Abreu F (1998). Risky choice and Weber’s Law. J Theor Biol 194: 289–298.
Kahneman D, Tversky A (2000) Choices Values and Frames. Cambridge University Press: Cambridge, UK.
Lesieur HR (1979). The compulsive gambler’s spiral of options and involvement. Psychiatry 42: 79–87.
Linnet J, Moller A, Peterson E, Gjedde A, Doudet D (2011). Inverse association between dopaminergic neurotransmission and Iowa Gambling Task performance in pathological gamblers and healthy controls. Scand J Psychol 52: 28–34.
Marsh B, Kacelnik A (2002). Framing effects and risky decisions in starlings. Proc Natl Acad Sci USA 99: 3352–3355.
Mayfield RD, Randall PK, Spirduso WW, Wilcox RE (1993). Selective D1 and D2 dopamine receptor antagonists produce differential effects on reaction time in the rat. Pharmacol Biochem Behav 46: 759–768.
Pucadyil TJ, Kalipatnapu S, Chattopadhyay A (2005). The serotonin(1A) receptor: a representative member of the serotonin receptor family. Cell Mol Neurobiol 25: 553–580.
Rivalan M, Ahmed SH, Dellu-Hagedorn F (2009). Risk-prone individuals prefer the wrong options on a rat version of the Iowa Gambling Task. Biol Psychiatry 66: 743–749.
Rogers RD, Wielenberg B, Wojtecki L, Elben S, Campbell-Meiklejohn D, Schnitzler A (2011). Deep brain stimulation of the subthalamic nucleus transiently enhances loss-chasing behaviour in patients with Parkinson’s disease. Exp Neurol 231: 181–189.
Scarf D, Miles K, Sloan A, Goulter N, Hegan M, Seid-Fatemi A et al (2011). Brain cells in the avian ‘prefrontal cortex’ code for features of slot-machine-like gambling. PLoS One 6: e14589.
Schultz W (2010). Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct 6: 24.
Seeman P, Ulpian C (1988). Dopamine D1 and D2 receptor selectivities of agonists and antagonists. Adv Exp Med Biol 235: 55–63.
Smee ML, Overstreet DH (1977). Biphasic effects of apomorphine on locomotor activity in rats. Commun Psychopharmacol 1: 99–104.
St Onge JR, Abhari H, Floresco SB (2011). Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci 31: 8625–8633.
Tremblay AM, Desmond RC, Poulos CX, Zack M (2010). Haloperidol modifies instrumental aspects of slot machine gambling in pathological gamblers and healthy controls. Addict Biol 16: 467–484.
van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ (2006). Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 187: 73–85.
Verbruggen F, Adams R, Chambers CD (2012). Proactive motor control reduces monetary risk taking in gambling. Psychol Sci 23: 805–815.
Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ et al (2010). Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron 65: 135–142.
Winstanley CA, Cocker PJ, Rogers RD (2011). Dopamine modulates reward expectancy during performance of a slot machine task in rats: evidence for a ‘near-miss’ effect. Neuropsychopharmacology 36: 913–925.
Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2004). Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology 29: 1331–1343.
Zack M, Poulos CX (2004). Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology 29: 195–207.
Zack M, Poulos C (2007). A D2 antagonist enhances the rewarding and priming effects of a gambling episode in pathological gamblers. Neuropsychopharmacology 32: 1678–1686.
Zeeb FD, Robbins TW, Winstanley CA (2009). Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology 34: 2329–2343.
Acknowledgements
This work was supported by an operating grant awarded to CAW from the Canadian Institutes for Health Research (CIHR). CAW also receives salary support through the Michael Smith Foundation for Health Research and the CIHR New Investigator Award program. We thank three anonymous reviewers for helpful contributions to the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
CAW has previously consulted for Theravance on an unrelated matter. The authors declare no conflict of interest or financial disclosures.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Rogers, R., Wong, A., McKinnon, C. et al. Systemic Administration of 8-OH-DPAT and Eticlopride, but not SCH23390, Alters Loss-Chasing Behavior in the Rat. Neuropsychopharmacol 38, 1094–1104 (2013). https://doi.org/10.1038/npp.2013.8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.8