Abstract
Studies suggest that heightened peripheral inflammation contributes to the pathogenesis of major depressive disorder. We investigated the effect of chronic social defeat stress, a mouse model of depression, on blood–brain barrier (BBB) permeability and infiltration of peripheral immune signals. We found reduced expression of the endothelial cell tight junction protein claudin-5 (Cldn5) and abnormal blood vessel morphology in nucleus accumbens (NAc) of stress-susceptible but not resilient mice. CLDN5 expression was also decreased in NAc of depressed patients. Cldn5 downregulation was sufficient to induce depression-like behaviors following subthreshold social stress whereas chronic antidepressant treatment rescued Cldn5 loss and promoted resilience. Reduced BBB integrity in NAc of stress-susceptible or mice injected with adeno-associated virus expressing shRNA against Cldn5 caused infiltration of the peripheral cytokine interleukin-6 (IL-6) into brain parenchyma and subsequent expression of depression-like behaviors. These findings suggest that chronic social stress alters BBB integrity through loss of tight junction protein Cldn5, promoting peripheral IL-6 passage across the BBB and depression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ménard, C., Pfau, M. L., Hodes, G. E. & Russo, S. J. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology 42, 62–80 (2017).
Kessler, R. C., Chiu, W. T., Demler, O., Merikangas, K. R. & Walters, E. E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey replication. Arch. Gen. Psychiatry 62, 617–627 (2005).
Seligman, F. & Nemeroff, C. B. The interface of depression and cardiovascular disease: therapeutic implications. Ann. NY Acad. Sci. 1345, 25–35 (2015).
Carney, R. M. & Freedland, K. E. Depression and coronary heart disease. Nat. Rev. Cardiol. 14, 145–155 (2017).
Wood, S. K. Individual differences in the neurobiology of social stress: implications for depression-cardiovascular disease comorbidity. Curr. Neuropharmacol. 12, 205–211 (2014).
Huffman, J. C., Celano, C. M., Beach, S. R., Motiwala, S. R. & Januzzi, J. L. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc. Psychiatry Neurol. 2013, 695925 (2013).
Hodes, G. E., Kana, V., Menard, C., Merad, M. & Russo, S. J. Neuroimmune mechanisms of depression. Nat. Neurosci. 18, 1386–1393 (2015).
Barnes, J., Mondelli, V. & Pariante, C. M. Genetic contributions of inflammation to depression. Neuropsychopharmacology 42, 81–98 (2017).
Dantzer, R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 29, 247–264 (2009).
Miller, A. H. & Raison, C. L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2016).
Hodes, G. E. et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. USA 111, 16136–16141 (2014).
Powell, N. D. et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA 110, 16574–16579 (2013).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
Wohleb, E. S., Powell, N. D., Godbout, J. P. & Sheridan, J. F. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 33, 13820–13833 (2013).
Weber, M. D., Godbout, J. P. & Sheridan, J. F. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology 42, 46–61 (2017).
Esposito, P. et al. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 888, 117–127 (2001).
Sántha, P. et al. Restraint stress-induced morphological changes at the blood-brain barrier in adult rats. Front. Mol. Neurosci. 8, 88 (2016).
Friedman, A. et al. Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response. Nat. Med. 2, 1382–1385 (1996).
Sharma, H. S. & Dey, P. K. Impairment of blood-brain barrier (BBB) in rat by immobilization stress: role of serotonin (5-HT). Indian J. Physiol. Pharmacol. 25, 111–122 (1981).
Niklasson, F. & Agren, H. Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biol. Psychiatry 19, 1183–1206 (1984).
Roszkowski, M. & Bohacek, J. Stress does not increase blood-brain barrier permeability in mice. J. Cereb. Blood Flow Metab. 36, 1304–1315 (2016).
Günzel, D. & Yu, A. S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 93, 525–569 (2013).
Nitta, T. et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 161, 653–660 (2003).
Meltzer, H., Vostanis, P., Ford, T., Bebbington, P. & Dennis, M. S. Victims of bullying in childhood and suicide attempts in adulthood. Eur. Psychiatry 26, 498–503 (2011).
Berton, O. & Nestler, E. J. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 7, 137–151 (2006).
Ménard, C., Hodes, G. E. & Russo, S. J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 321, 138–162 (2016).
Golden, S. A., Covington, H. E. III, Berton, O. & Russo, S. J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 6, 1183–1191 (2011).
Russo, S. J. & Nestler, E. J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–625 (2013).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 (2016).
Hodes, G. E. et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. 35, 16362–16376 (2015).
Golden, S. A. et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat. Med. 19, 337–344 (2013).
Campbell, M. et al. Systemic low-molecular weight drug delivery to pre-selected neuronal regions. EMBO Mol. Med. 3, 235–245 (2011).
McKim, D.B. et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry https://doi.org/10.1038/mp.2017.64 (2017).
Saederup, N. et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One 5, e13693 (2010).
Mizutani, M. et al. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J. Immunol. 188, 29–36 (2012).
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Maes, M. et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9, 853–858 (1997).
Dowlati, Y. et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457 (2010).
Kiraly, D. D. et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl. Psychiatry 7, e1065 (2017).
Coppen, A. J. Abnormality of the blood-cerebrospinal fluid barrier of patients suffering from a depressive illness. J. Neurol. Neurosurg. Psychiatry 23, 156–161 (1960).
Hambardzumyan, D., Gutmann, D. H. & Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27 (2016).
Shichita, T. et al. Pivotal role of cerebral interleukin-17-producing γδT cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950 (2009).
Golden, S. A. et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692 (2016).
Mishra, V. et al. Primary blast causes mild, moderate, severe and lethal TBI with increasing blast overpressures: experimental rat injury model. Sci. Rep. 6, 26992 (2016).
Keaney, J. et al. Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci. Adv. 1, e1500472 (2015).
Campbell, M. et al. RNAi-mediated reversible opening of the blood-brain barrier. J. Gene Med. 10, 930–947 (2008).
Doyle, S. L. et al. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci. Transl. Med. 6, 230ra44 (2014).
Wälchli, T. et al. Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain. Nat. Protoc. 10, 53–74 (2015).
Blank, T. et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity 44, 901–912 (2016).
Janssen, W. G. et al. Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp. Neurol. 191 (Suppl. 1), S28–S44 (2005).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Acknowledgements
The authors thank A. Keller for advice on BBB-related studies and the Center for Comparative Medicine and Surgery housing facilities staff for their work and support. This research was supported by Mental Health grants RO1 MH090264 (S.J.R.), P50 MH096890 (S.J.R.), P50 AT008661-01 (S.J.R.), RO1 MH114882 (S.J.R.), RO1 MH104559 (S.J.R. and M.M.), NIH/NHLBI P01 HL131478 (Z.A.F.), T32 MH087004 (M.L.P., M.H. and M.F.), T32 MH096678 (M.L.P.), F30 MH100835 (M.H.) and F31 MH105217 (M.L.P.), a Janssen/IMHRO Rising Star Translational Research Award (S.J.R.), a Swiss National Science Foundation Advanced Postdoc Mobility Fellowship (V.K.) and a Brain and Behavior Research Foundation NARSAD Young Investigator Award (G.E.H.). C.M. is supported by a Brain and Behavior Research Foundation NARSAD Young Investigator Grant sponsored by the P&S Fund.
Author information
Authors and Affiliations
Contributions
C.M. and S.J.R. designed the study and wrote the manuscript. C.M., M.L.P., G.E.H., A.T., M.E.F., H.A., K.B.L., Z.S.L., S.A.G. and M.H. performed stereotaxic surgeries, tissue collection and behavioral manipulations and analyzed data. V.K. performed and analyzed flow experiments and Ccr2 RFP::Cx3cr1 GFP mouse immunostaining. V.K. and M.M. provided Ccr2 RFP::Cx3cr1 GFP mice and provided advice on BBB- and immune-related studies. V.X.W., Z.A.F. and C.Y.T. designed, performed and analyzed magnetic resonance imaging scans. S.B. advised on analysis approaches and analyzed data. W.G.J. prepared and imaged transmission electron microscopy samples. B.L., E.M.P., C.T. and G.T. provided post-mortem human tissue samples. M.C. provided viral vectors and advised on viral studies. All the authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated Supplementary Information
Supplementary Figure 1 Behavioral phenotype of quantitative PCR (qPCR) experiments and Cldn5 expression in other brain regions or animal model of stress
Stress-susceptible (SS) mice spent less time in the interaction zone (***p < 0.0001) (A) and more time in the corners (***p < 0.0001) (B) when the social target (aggressor, AGG) was present compared to unstressed control (CTRL) and resilient (RES). However no significant difference was observed when the social target was absent. C) Stressed mice traveled less distance than CTRL when the social target was either present (***p = 0.0004) or absent (***p = 0.0004) but no difference was observed between SS and RES groups. D) Heat maps of gene expression related to endothelial cells and tight junctions normalized to blood vessel marker pecam1. E) Normalization to pecam1 confirmed specific reduction of cldn5 expression in the nucleus accumbens (NAc) of SS mice (***p = 0.0009). Cldn5 mRNA expression was reduced in the hippocampus (G) of both SS and RES mice after 10-day chronic social defeat stress (CSDS) while no change was measured in the prefrontal cortex (PFC) (F) or hypothalamus (H). Gapdh was used as housekeeping gene for hippocampus (HIPP), prefrontal cortex (PFC) and hypothalamus studies. I) Six days of chronic variable stress (CVS) did not modify cldn5 mRNA expression however, after 28 days of CVS, cldn5 mRNA level was significantly reduced in the NAc of male C57Bl/6 mice (J). Gapdh was used as housekeeping gene for CVS studies. Data represent mean ± SEM, number of animal (n) is indicated on graphs. Unpaired t-test for CUS-related qPCR experiments and one-way ANOVA for other graphs followed by Bonferroni’s multiple comparison test, *p < 0.05; **p < 0.01; ***p < 0.001
Supplementary Figure 2 Behavioral phenotype of immunohistochemistry (IHC) experiments and assessment of Cldn5 endothelial cell specificity and stress-induced loss
SS mice spent less time in the interaction zone (**p = 0.0058) (A) and more time in the corners (**p = 0.0090) (B) when the social target (AGG) was present compared to unstressed CTRL and RES mice. SS mice spent more time in the interaction zone when the social target was absent (*p = 0.0124) (A) but no significant difference was observed for time spent in corners. C) Stressed mice traveled less distance than CTRL when the social target was either present (**p = 0.0024) or absent (*p = 0.0133) but no difference was observed between SS and RES groups. D) Cldn5 mRNA expression is selectively expressed in endothelial cells in the adult mouse and human brain according to RNA-sequencing transcriptome databases29,30. E) Cldn5 protein level was enriched five-fold in half-brain capillary extraction when compared to whole cell homogenates (***p < 0.0001). Purity of the extraction was validated with astrocyte (glial fibrillary acidic protein, gfap) and neuronal (neun) markers. Representative blots in triplicate are shown. F) Following 10-day CSDS, no difference was observed in CD31, occluding or ZO-1 protein levels in the NAc of SS or RES mice. Data represent mean ± SEM, number of animals (n) is indicated on graphs. Unpaired t-test for Western Blot analyses, Pearson’s correlation for tight junction protein level vs social interaction (SI) ratio and one-way ANOVA for other graphs followed by Bonferroni’s multiple comparison test, *p < 0.05; **p < 0.01; ***p < 0.001
Supplementary Figure 3 Tight junction protein levels in the hippocampus (HIPP) and prefrontal cortex (PFC) following 10-d CSDS
A) Cldn5 (*p = 0.0236) and occludin (*p = 0.0327) protein levels were significantly higher in RES mice when compared to SS, but not unstressed control mice. Occludin protein level was positively correlated with SI ratio (*p = 0.0486). No difference was observed for ZO-1 or the endothelial cell marker CD31. B) 10-day CSDS had no effect on tight junction protein levels or CD31 in the PFC. Scale bar at 200 µm. Images corresponded to flattened 1-µm-thick z-stacks at 40x magnification. Data represent mean ± SEM, number of animals (n) is indicated on graphs. One-way ANOVA followed by Bonferroni’s multiple comparison test and Pearson’s correlation for tight junction protein level vs SI ratio, *p < 0.05
Supplementary Figure 4 Blood vessel and capillary morphology in the NAc and PFC of CTRL, SS and RES mice
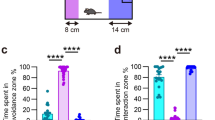
A) Detailed behavioral phenotyping of the mice imaged with transmission electron microscopy 48 hours after the last defeat. SS mice SI ratio was lower than CTRL and RES mice (***p = 0.0001) as they spent less time in the interaction zone (***p < 0.0001) (A) and more time in the corners (***p = 0.0001) (B) when the social target (AGG) was present. C) No difference was observed between groups for distance traveled. D) Representative pictures of large blood vessels and capillaries in the NAc of CTRL, SS and RES mice. Mice were injected with horseradish peroxidase (HRP) that was allowed to circulate for 2 hours before 20-min perfusion and fixation. Scale bar is set at 2 µm or 500 nm. E) No significant difference was measured for tight junction discontinuity in the PFC of SS or RES mice when compared to CTRL mice. Scale bar is set at 2 µm or 500 nm, 15-24 tight junctions/mouse for three mice/group. Data represent mean ± SEM, number of animals (n) is indicated on graphs. One-way ANOVA followed by Bonferroni’s multiple comparison test, **p < 0.01, ***p < 0.001
Supplementary Figure 5 Behavioral phenotype of SS mice treated chronically with vehicle or the antidepressant imipramine and CLDN5 expression in MDD patients and cocaine users
A) Timeline showing mice were exposed to 10-day CSDS then screened for behavioral phenotype 24 hours later. Vehicle-treated SS mice spent less time in the interaction zone (B) and more time in the corners (C) when the social target (AGG) was present when compared to the other groups, including imipramine-treated SS mice. No difference was observed when the social target was absent. D) Locomotion was similar between all groups despite the presence or absence of the social target. E) Acute treatment with the antidepressant imipramine is not sufficient to rescue cldn5 loss after 10-day CSDS. F) No significant difference was measured for GAPDH in NAc postmortem tissue from healthy controls (CTRL) or MDD patients with (MDD, AD + ) or without (MDD, AD-) antidepressant treatment at the time of death. G) CLDN5 expression in the HIPP and PFC (normalized to GAPDH housekeeping gene) was not significantly different in MDD patients treated or untreated with antidepressants when compared to healthy controls. H) CLDN5 expression is unchanged in cocaine users when compared to healthy controls (normalized to GAPDH housekeeping gene). Data represent mean ± SEM, number of animals or subjects (n) is indicated on graphs. Unpaired t-test for acute treatment and cocaine users, one-way ANOVA for MDD cohorts and two-way ANOVA for other graphs followed by Bonferroni’s multiple comparison test, *p < 0.05
Supplementary Figure 6 Gene expression in NAc of AAV-shRNA-Cldn5-injected mice, supplementary SI behaviors and anxiety test results
A) No significant change was observed at mRNA level in the NAc of AAV-shRNA versus AAV-shRNA-cldn5 for other claudins (cdn1, cldn3, cdn12), cytoskeletal regulators (rac1) or markers of endothelial cells (pecam1), pericytes (pdgfrβ), astrocytes (gfap, s100β) and microglia (iba1) when normalized to the housekeeping gene gapdh. B) CD31 protein level was similar in the NAc of AAV-shRNA-cldn5 and AAV-shRNA-injected mice. C) Stressed AAV-shRNA-injected mice spent less time in the interaction zone and more time in the corners when the social target (AGG) was present. No difference was observed between mice groups for time spent in the interaction zone or corners when the social target was absent or for overall locomotion. D) No significant difference was measured between groups for latency to eat in the novelty-suppressed feeding test (left) or home cage (right). Virus injection in the NAc had no effect on anxiety as measured with the elevated plus maze (E) and open field (F) tests. Data represent mean ± SEM, number of animals (n) is indicated on graphs. Unpaired t-test for virus validation and two-way ANOVA followed by Bonferroni’s multiple comparison test for behavioral experiments, *p < 0.05, ***p < 0.001
Supplementary Figure 7 Experimental timeline for virally mediated HIPP injections and rescue experiment, virus validation and behaviors
Experimental timeline (A) and virus validation (qPCRs, **p = 0.0039, Western Blots, ***p = 0.0006) (B) for AAV-shRNA and AAV-shRNA-cldn5 injections in the HIPP. Full-length blots are included in Supplementary Fig. 12. Downregulation of cldn5 expression had no effect in the splash (C) and sucrose preference tests (D). However mice injected with AAV-shRNA-cldn5 spent more time immobile in the forced swim test (E) and stressed AAV-shRNA-cldn5 mice displayed social avoidance (F). Representative heat maps are shown on the right. G) Experimental timeline of the control cohort for rescue experiment. H) Removal of Dox from the water allowed recovery of cldn5 expression at both mRNA (p = 0.9473) and protein (p = 0.2077) levels in the rescue cohort. Full-length blots are included in Supplementary Fig. 12. I) Conversely, downregulation of cldn5 mRNA (**p = 0.0020) and protein (*p = 0.0116) levels was confirmed in the cohort remaining on Dox. Full-length blots are included in Supplementary Fig. 12. G) Stressed AAV-shRNA-cldn5 mice that remained on dox treatment displayed depression-like behaviors in forced swim (**p = 0.0015) and sucrose preference (**p = 0.0028) tests before (J) and after 5-day rest as measured with sucrose preference (***p = 0.0002) and social interaction tests (**p = 0.0028) (K). Representative heat maps are shown on the left. Data represent mean ± SEM, number of animals (n) is indicated on graphs. Two-way ANOVA followed by Bonferroni’s multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001
Supplementary Figure 8 Gd-DTPA and cadaverine Alexa Fluor-555 experiments
A cohort of mice was first behaviorally characterized for MRI studies. SS mice spent less time in the interaction zone (A) and more time in the corners (B) when the social target (AGG) was present when compared to CTRL and RES mice. No difference was observed when the social target was absent. Locomotion was similar in SS and RES mice (C). D) Higher Gd-DTPA signal was detected in different brain regions, including the NAc, in SS mice and negatively correlated with SI ratio. E) Gd-DTPA level was higher in the hippocampus of SS mice versus RES mice and significantly correlated with social avoidance. F) Conversely, no difference was measured between groups for the PFC. A second cohort of mice was behaviorally characterized to assess BBB permeability to cadaverine Alexa Fluor-555. After 10-day CSDS, SS mice spent less time in the interaction zone (G) and more time in the corners (H) versus CTRL and RES groups when the social target was present (AGG). No difference was observed when the social target was absent. I) Stressed mice traveled less distance compared to CTRL when the social target was either present or absent but no significant difference was measured between SS and RES groups. Cadaverine level is significantly correlated with social avoidance in the nucleus accumbens (NAc) (J) and hippocampus (K). L) No difference was measured between groups for the PFC. Data are presented as mean ± SEM, number of animals (n) is indicated on graphs. Correlations were evaluated with Pearson’s correlation coefficient, one-way ANOVA followed by Bonferroni’s multiple comparison test for other graphs, *p < 0.05; **p < 0.01; ***p < 0.001
Supplementary Figure 9 Supplementary behavioral data for Evans blue (EB) extravasation and Ccr2 RFP::Cx3cr1 GFP mice after CSDS
A cohort of mice was behaviorally characterized to assess BBB permeability to circulating EB. SS mice spent less time in the interaction zone (A) and more time in the corners (B) when the social target (AGG) was present when compared to CTRL and RES mice. SS mice spent more time in the interaction zone when the social target was absent (A) but no difference was observed for the corners (B). C) Stressed mice displayed less locomotion versus unstressed CTRL when the social target was either present or absent. However no significant difference was measured between SS and RES groups. D) EB level in the NAc was significantly correlated with social avoidance. E) EB could be detected in hippocampus blood vessels 10 min after the retro-orbital injection was performed (left). No EB extravasation was observed after 16-h circulation followed by 5-min perfusion in the hippocampus (right). F) Similarly, EB was detectable in PFC blood vessels 10-min after the injection but not after 16-h circulation and 5-min perfusion. No difference was measured for the hippocampus (G) or PFC (H) after EB extraction. I) C-C chemokine receptor 2 (ccr2) mRNA expression is specifically elevated in the NAc of SS mice after 10-day CSDS and correlated with social avoidance. J) Stressed ccr2 RFP:: cx3cr1 GFP mice spent less time in the interaction zone when the social target (AGG) was present. Lower overall locomotion was also observed in stressed ccr2 RFP:: cx3cr1 GFP mice when compared to unstressed controls when the AGG was absent. K) Flow cytometry gating strategy for ccr2 RFP monocytes and cx3cr1 GFP microglia. L) No difference was measured between groups for percentage (%) of ccr2-/cx3cr1+ cells (microglia). M) Immunohistochemical analysis of ccr2RFP+ monocytes shows that they accumulate within blood vessels of the NAc (left) and lateral ventricle (right), but not in the parenchyma. Scale bar at 100 µm (50 µm for the insets). Data represent mean ± SEM, number of animals (n) is indicated on graphs. Correlations were evaluated with Pearson’s correlation coefficient, unpaired t-test for ccr2 RFP :: cx3cr1 GFP mice and one-way ANOVA followed by Bonferroni’s multiple comparison test for other graphs, *p < 0.05; **p < 0.01, ***p < 0.001
Supplementary Figure 10 Behavioral data for IL-6 ELISA, IL-6–biotin passage into the parenchyma and NAc IL-6 versus saline infusion
A) Following 10-day CSDS, blood serum and NAc, HIPP and PFC punches were collected to assess IL-6 protein level 48 h after the last defeat. SS mice spent less time in the interaction zone (***p < 0.0001) and more time in the corners (***p < 0.0001) when the aggressor (AGG) was present, No difference was observed when the AGG was absent or between SS and RES mice for overall locomotion. B) IL-6 protein was barely detectable in the HIPP and PFC of CTRL, SS and RES mice. C) Circulating IL-6 level is increased 20 min after recombinant IL-6 i.p. injections. D) SI ratio of mice retro-orbitally injected with biotinylated IL-6 (IL6-biotin) 24h after the SI test (48h after the last defeat or 10-day CSDS). E) SS mice spent less time in the interaction zone, and more time in the corners when the social target was present compared to CTRL and RES animals. No difference was observed for locomotion. F) Minute amount of IL6-biotin-Neutravidin-Oregon488 was detectable in the HIPP of SS mice. None was detectable in the PFC (G). H) Mice administered direct infusion of IL-6 into the NAc spent less time in the interaction zone when the social target AGG was present, but not absent, when compared to the saline group. No difference was observed for the time spent in corners (I) or locomotion (J). Data represent mean ± SEM, number of animals (n) is indicated on graphs. One-way ANOVA followed by Bonferroni’s multiple comparison test for (A-D) and two-way ANOVA followed by Bonferroni’s multiple comparison test for other graphs, *p < 0.05; **p < 0.01
Supplementary Figure 11 Social stress induces neurovascular pathology and BBB leakiness promoting depression-like behaviors
10-day chronic social defeat stress (CSDS) induces loss of Cldn5 expression at mRNA and protein levels, leading to abnormalities in blood vessel morphology and increased BBB permeability in stress-susceptible (SS) mice. These molecular and cellular changes are associated with depression-like behavior such as social avoidance, anhedonia, despair and lack of self-care. Conversely, mice resilient (RES) to CSDS display normal social and stress coping behaviors and neurovascular features similar to unstressed controls (CTRL)
Supplementary Figure 12 Full-length blots of cropped blots in Supplementary Fig. 7
A) Full-length Western blot for cldn5 (top) and actin (bottom) after AAV-shRNA-cldn5 viral injection in the hippocampus (HIPP). B) Full-length Western blot for cldn5 (top) and actin (bottom) after doxycycline (Dox) was removed from the drinking water (Rescue – no Dox) in mice injected with AAV-shRNA-cldn5 in the nucleus accumbens (NAc). C) Full-length Western Blot for cldn5 (top) and actin (bottom) in the NAc of AAV-shRNA-cldn5-injected mice remaining on Dox for the entire experiment
Supplementary material
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1–4
Rights and permissions
About this article
Cite this article
Menard, C., Pfau, M.L., Hodes, G.E. et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20, 1752–1760 (2017). https://doi.org/10.1038/s41593-017-0010-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-017-0010-3
This article is cited by
-
Chronic restraint stress promotes oral squamous cell carcinoma development by inhibiting ALDH3A1 via stress response hormone
BMC Oral Health (2024)
-
Microbial composition, functionality, and stress resilience or susceptibility: unraveling sex-specific patterns
Biology of Sex Differences (2024)
-
The therapeutic potential of probucol and probucol analogues in neurodegenerative diseases
Translational Neurodegeneration (2024)
-
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
Nature Reviews Gastroenterology & Hepatology (2024)
-
Enhanced fear memory after social defeat in mice is dependent on interleukin-1 receptor signaling in glutamatergic neurons
Molecular Psychiatry (2024)