Abstract
Objective:
To investigate the neural basis of the abnormal eating behaviour in Prader–Willi syndrome (PWS), using brain imaging. We predicted that the satiety response in those with PWS would be delayed and insensitive to food intake.
Design and participants:
The design of this study was based on a previous investigation of the neural activation associated with conditions of fasting and food intake in a nonobese, non-PWS group. The findings were used to generate specific hypotheses regarding brain regions of interest for the current study, in which 13 adults with PWS took part (mean±s.d. age=29±6; BMI=31.5±5.1; IQ=71±8, six were female).
Measurements:
Regional cerebral blood flow was measured using positron emission tomography in three sessions: one following an overnight fast and two following disguised energy controlled meals of similar volume and appearance – one of 1674 kJ (400 kcal) and another of 5021 kJ (1200 kcal). Subjective ratings of hunger, fullness and desire to eat, and blood plasma levels of glucose, insulin, leptin, ghrelin and PYY were measured before and after each imaging session.
Results:
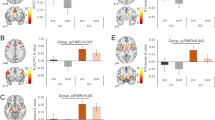
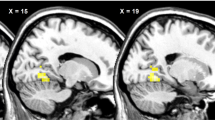
The neural representation of hunger, after an overnight fast, was similar to that found in nonobese individuals in the control study. In contrast, after food intake, the patterns of neural activation previously associated with satiety were not found, even after the higher-energy load. Lateral and medial orbitofrontal cortical activation was associated with consumption of the 400- and 1200-kcal meals, respectively. The medial orbitofrontal activation, however, was only found in those who had shown a large percentage change in fullness ratings following the higher-energy meal.
Conclusion:
We conclude that there is a dysfunction in the satiety system in those with PWS. These findings suggest that brain regions associated with satiety are insensitive even to high-energy food intake in those with the syndrome. This may be the neural basis of the hyperphagia seen in PWS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H . Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet 2001; 38: 792–798.
Davies PS, Joughin C . Using stable isotopes to assess reduced physical activity of individuals with Prader–Willi syndrome. Am J Ment Retard 1993; 98: 349–353.
Pipes PL, Holm VA . Weight control of children with Prader–Willi syndrome. J Am Diet Assoc 1973; 62: 520–524.
Zipf WB, Berntson GG . Characteristics of abnormal food-intake patterns in children with Prader–Willi syndrome and study of effects of naloxone. Am J Clin Nutr 1987; 46: 277–281.
Holland AJ, Treasure J, Coskeran P, Dallow J, Milton N, Hillhouse E . Measurement of excessive appetite and metabolic changes in Prader–Willi syndrome. Int J Obes Relat Metab Disord 1993; 17: 527–532.
Lindgren AC, Barkeling B, Hagg A, Ritzen EM, Marcus C, Rossner S . Eating behavior in Prader–Willi syndrome, normal weight, and obese control groups. J Pediatr 2000; 137: 50–55.
Goldstone AP . Prader–Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab 2004; 15: 12–20.
Swaab DF . Prader–Willi syndrome and the hypothalamus. Acta Paediatr Suppl 1997; 423: 50–54.
Shapira NA, Lessig MC, He AG, James GA, Driscoll DJ, Liu Y . Satiety dysfunction in Prader–Willi syndrome demonstrated by fMRI. J Neurol Neurosurg Psychiatry 2005; 76: 260–262.
Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999; 48: 1801–1806.
Liu Y, Gao JH, Liu HL, Fox PT . The temporal response of the brain after eating revealed by functional MRI. Nature 2000; 405: 1058–1062.
Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM . Neural contributions to the motivational control of appetite in humans. Eur J Neurosci 2004; 20: 1411–1418.
Tataranni PA, DelParigi A . Functional neuroimaging: a new generation of human brain studies in obesity research. Obes Rev 2003; 4: 229–238.
Small DM . Toward an understanding of the brain substrates of reward in humans. Neuron 2002; 33: 668–671.
Kringelbach ML . Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience 2004; 126: 807–819.
Berthoud HR . Mind versus metabolism in the control of food intake and energy balance. Physiol Behav 2004; 81: 781–793.
Burton MJ, Rolls ET, Mora F . Effects of hunger on the responses of neurons in the lateral hypothalamus to the sight and taste of food. Exp Neurol 1976; 51: 668–677.
Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS . Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 1999; 20: 68–100.
Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG . Central nervous system control of food intake. Nature 2000; 404: 661–671.
Schultz W, Tremblay L, Hollerman JR . Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex 2000; 10: 272–284.
Critchley HD, Rolls ET . Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J Neurophysiol 1996; 75: 1673–1686.
O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport 2000; 11: 893–897.
Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 2004; 21: 1790–1797.
Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 1999; 96: 4569–4574.
Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M . Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 2001; 124 (Part 9): 1720–1733.
Morris JS, Dolan RJ . Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci 2001; 21: 5304–5310.
Tremblay L, Schultz W . Relative reward preference in primate orbitofrontal cortex. Nature 1999; 398: 704–708.
Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC . Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci 2003; 23: 9632–9638.
Gottfried JA, O'Doherty J, Dolan RJ . Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 2003; 301: 1104–1107.
Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G . Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci 2003; 23: 11078–11084.
Lucignani G, Panzacchi A, Bosio L, Moresco RM, Ravasi L, Coppa I et al. GABA A receptor abnormalities in Prader–Willi syndrome assessed with positron emission tomography and flumazenil. Neuroimage 2004; 22: 22–28.
Bender DA . Introduction to Nutrition and Metabolism. 2nd edn. Taylor & Francis: London, 1997.
Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 2003; 349: 941–948.
Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR . Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985; 89: 1070–1077.
Goldstone AP, Patterson M, Kalingag N, Ghatei MA, Brynes AE, Bloom SR et al. Fasting and post-prandial hyperghrelinemia in Prader–Willi syndrome is partially explained by hypoinsulinemia, and is not due to PYY deficiency or seen in hypothalamic obesity due to craniopharyngioma. J Clin Endocrinol Metab 2005; 90: 2681–2690.
Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR . Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab 2005; 90: 2205–2211.
Nelson M, Atkinson M, Meyer J . Food Portion Sizes: A Photographic Atlas. Food Standards Agency (MAFF), London, 1997.
Russell H, Oliver C . The assessment of food-related problems in individuals with Prader–Willi syndrome. Br J Clin Psychol 2003; 42 (Part 4): 379–392.
Brett M, Bloomfield P, Brooks D, Stein J, Grasby P . Scan order effects in PET activation studies are caused by motion artefact. Neuroimage 1999; 9: S56.
LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM . Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci 2001; 115: 493–500.
Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 2002; 8: 643–644.
DelParigi A, Tschop M, Heiman ML, Salbe AD, Vozarova B, Sell SM et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader–Willi syndrome. J Clin Endocrinol Metab 2002; 87: 5461–5464.
Haqq AM, Farooqi IS, O'Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader–Willi syndrome. J Clin Endocrinol Metab 2003; 88: 174–178.
Tan TM, Vanderpump M, Khoo B, Patterson M, Ghatei MA, Goldstone AP . Somatostatin infusion lowers plasma ghrelin without reducing appetite in adults with Prader–Willi syndrome. J Clin Endocrinol Metab 2004; 89: 4162–4165.
Haqq AM, Stadler DD, Rosenfeld RG, Pratt KL, Weigle DS, Frayo RS et al. Circulating ghrelin levels are suppressed by meals and octreotide therapy in children with Prader–Willi syndrome. J Clin Endocrinol Metab 2003; 88: 3573–3576.
Kringelbach ML, Rolls ET . The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004; 72: 341–372.
Butler JV, Whittington JE, Holland AJ, Boer H, Clarke D, Webb T . Prevalence of, and risk factors for, physical ill-health in people with Prader–Willi syndrome: a population-based study. Dev Med Child Neurol 2002; 44: 248–255.
Acknowledgements
We thank the participants with PWS and their carers. We would also like to thank Daniel Bor, John A Parkinson, Joyce Whittington and Angela C Roberts for helpful advice and discussion, and the staff at the Wolfson Brain Imaging Centre and Department of Clinical Biochemistry, Cambridge. This research was funded by a bequest to the PWSA (UK), and by the MRC Cognition and Brain Sciences Unit, Cambridge. Food supplements were provided by SHS International Ltd. The study was conceived and designed by AJ Holland, AM Owen, and EC Hinton. The study was carried out by EC Hinton, who co-wrote the paper with AJ Holland, with contributions from AM Owen. MSN Gellatly gave dietetic advice for designing the breakfasts, and S Soni carried out cognitive assessments and medical supervision for PET. Michael Patterson and Professor Ghatei conducted and co-ordinated the ghrelin and PYY assays.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hinton, E., Holland, A., Gellatly, M. et al. Neural representations of hunger and satiety in Prader–Willi syndrome. Int J Obes 30, 313–321 (2006). https://doi.org/10.1038/sj.ijo.0803128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803128
Keywords
This article is cited by
-
Prader-Willi syndrome mental health research strategy workshop proceedings: the state of the science and future directions
Orphanet Journal of Rare Diseases (2016)
-
Deactivation of the left dorsolateral prefrontal cortex in Prader–Willi syndrome after meal consumption
International Journal of Obesity (2016)
-
Importance of reward and prefrontal circuitry in hunger and satiety: Prader–Willi syndrome vs simple obesity
International Journal of Obesity (2012)
-
Appetite hormones and the transition to hyperphagia in children with Prader-Willi syndrome
International Journal of Obesity (2012)
-
Development of the eating behaviour in Prader–Willi Syndrome: advances in our understanding
International Journal of Obesity (2011)