Abstract
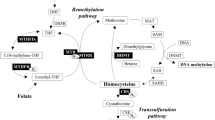
Elevated plasma homocysteine concentration has been suggested as a risk factor for schizophrenia, but the results of epidemiological studies have been inconsistent. The most extensively studied genetic variant in the homocysteine metabolism is the 677C>T polymorphism in the methylenetetrahydrofolate reductase (MTHFR) gene, resulting in reduced enzyme activity and, subsequently, in elevated homocysteine. A meta-analysis of eight retrospective studies (812 cases and 2113 control subjects) was carried out to examine the association between homocysteine and schizophrenia. In addition, a meta-analysis of 10 studies (2265 cases and 2721 control subjects) on the homozygous (TT) genotype of the MTHFR 677C>T polymorphism was carried out to assess if this association is causal. A 5 μmol/l higher homocysteine level was associated with a 70% (95% confidence interval, CI: 27–129) higher risk of schizophrenia. The TT genotype was associated with a 36% (95% CI: 7–72) higher risk of schizophrenia compared to the CC genotype. The performed meta-analyses showed no evidence of publication bias or excessive influence attributable to any given study. In conclusion, our study provides evidence for an association of homocysteine with schizophrenia. The elevated risk of schizophrenia associated with the homozygous genotype of the MTHFR 677C>T polymorphism provides support for causality between a disturbed homocysteine metabolism and risk of schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gottesman II . Schizophrenia epigenesis: past, present, and future. Acta Psychiatr Scand 1994; 90: 26–33.
Portin P, Alanen YO . A critical review of genetic studies of schizophrenia. II. Molecular genetic studies. Acta Psychiatr Scand 1997; 95: 73–80.
Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M . The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch Gen Psychiatry 1998; 55: 67–74.
Kety SS . Schizophrenic illness in the families of schizophrenic adoptees: findings from the Danish national sample. Schizophr Bull 1998; 14: 217–222.
Harrison PJ, Weinberger DR . Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 2005; 10: 40–68.
Osmond H, Smythies J . Schizophrenia. A new approach. J Mental Sci 1952; 98: 309–315.
Smythies JR . Biochemistry of schizophrenia. Postgrad Med J 1963; 39: 26–33.
Scott JM, Weir DG . Folic acid, homocysteine, and one-carbon metabolism: a review of the essential biochemistry. J Cardiovasc Risk 1998; 5: 223–227.
Pollin W, Cardon PV, Kety SS . Effects of amino acid feedings in schizophrenic patients treated with iproniazid. Science 1961; 133: 104–105.
Cohen SM, Nichols A, Wyatt R, Pollin W . The administration of methionine to chronic schizophrenic patients: A review of ten studies. Biol Psychiatry 1974; 8: 209–225.
Antun FT, Kurkjian R . Demethylation of C14,2,3,4-trimethoxyphenethylamine in schizophrenics before and after L-methionine loading. Br J Psychiatry 1982; 140: 611–614.
Sargent III T, Kusubov N, Taylor SE, Budinger TF . Tracer kinetic evidence for abnormal methyl metabolism in schizophrenia. Biol Psychiatry 1992; 32: 1078–1090.
Smythies JR, Gotfries CG, Regland B . Disturbances of one-carbon metabolism in neuropsychiatric disorders: a review. Biol Psychiatry 1997; 41: 230–233.
Freeman JM, Finkelstein JD, Mudd SH . Folate-responsive homocysteinuria and ‘schizophrenia’: A defect in methylation due to deficient 5,10-methylenetetrahydrofolate reductase activity. N Engl J Med 1975; 292: 491–496.
Goyette P, Sumner JS, Milos R, Duncan AMW, Rosenblatt DS, Matthews RG et al. Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification. Nat Genet 1994; 7: 195–200.
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995; 10: 111–113.
Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Redlund M et al. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world-wide. J Med Genet 2003; 40: 619–625.
Brattstrom L, Wilcken DEL, Ohrvik J, Brudin L . Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation 1998; 98: 2520–2526.
Guenther BD, Sheppard CA, Tran P, Rozen R, Matthews RG, Ludwig ML . The structure and properties of methylenetetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia. Nat Struct Biol 1999; 6: 359–365.
Van der Put NM, Steegers-Theunissen RP, Frosst P, Trijbels FJ, Eskes TK, van den Heuvel LP et al. Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet 1995; 346: 1070–1071.
Hustad S, Ueland PM, Vollset SE, Zhang Y, Bjorke-Monsen AL, Schneede J . Riboflavin as a determinant of total plasma homocysteine: effect modification by methylenetetrahydrofolate reductase C677T polymorphism. Clin Chem 2000; 46: 1065–1071.
Muntjewerff JW, Hoogendoorn MLC, Kahn RS, Sinke RJ, Den Heijer M, Kluijtmans LAJ et al. Hyperhomocysteinemia, methylenetetrahydrofolate reductase 677TT genotype, and the risk for schizophrenia. A Dutch population based case-control study. Am J Med Genet B Neuropsychiatr Genet 2005; 135: 69–72.
Woolf B . On estimating the relation between blood group and disease. Ann Hum Genet 1955; 19: 251–253.
Boushey CJ, Beresford SAA, Omenn GS, Motulsky AG . A quantative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 1995; 274: 1049–1057.
Wald DS, Law M, Morris JK . Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002; 325: 1202–1208.
Den Heijer M, Lewington S, Clarke R . Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost 2005; 3: 292–299.
DerSimonian R, Laird N . Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188.
Egger M, Davey Smith G, Schneider M, Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
Regland B, Johansson BV, Grenfeldt B, Hjelmgren LT, Medhus M . Homocysteinemia is a common feature of schizophrenia. J Neural Transm 1995; 100: 165–169.
Susser E, Brown AS, Klonowski E, Allen RH, Lindenbaum J . Schizophrenia and impaired homocysteine metabolism: a possible association. Biol Psychiatry 1998; 44: 141–143.
Virgos C, Martorell L, Simó JM, Valero J, Figuera L, Joven J et al. Plasma homocysteine and methylenetetrahydrofolate reductase C677T gene variant: lack of association with schizophrenia. Neuroreport 1999; 10: 2035–2038.
Levine J, Stahl Z, Sela BA, Gavendo S, Ruderman V, Belmaker RH . Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry 2002; 159: 1790–1792.
Muntjewerff JW, Van der Put N, Eskes T, Ellenbroek B, Steegers E, Blom H et al. Homocysteine metabolism and B-vitamins in schizophrenic patients: low plasma folate as a possible independent risk factor for schizophrenia. Psychiatry Res 2003; 121: 1–9.
Applebaum J, Shimon H, Sela BA, Belmaker RH, Levine J . Homocysteine levels in newly admitted schizophrenic patients. J Psychiatr Res 2004; 38: 413–416.
Goff DC, Bottiglieri T, Arning E, Shih V, Freudenreich O, Evins AE et al. Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry 2004; 161: 1705–1708.
Arinami T, Yamada N, Yamakawa-Kobayashi K, Hamaguchi H, Toru M . Methylenetetrahydrofolate reductase variant and schizophrenia/depression. Am J Med Genet 1997; 74: 526–528.
Kunugi H, Fukuda R, Hattori M, Kato T, Tatsumi M, Sakai T et al. C677T polymorphism in methylenetetrahydrofolate reductase gene and psychoses. Mol Psychiatry 1998; 3: 435–437.
Joober R, Benkelfat C, LaI S, Bloom D, Labelle A, Lalonde P et al. Association between the methylenetetrahydrofolate reductase 677C>T missense mutation and schizophrenia. Mol Psychiatry 2000; 5: 323–326.
Sazci A, Ergül E, Güzelhan Y, Kaya G, Kara I . Methylenetetrahydrofolate reductase gene polymorphism in patients with schizophrenia. Mol Brain Res 2003; 117: 104–107.
Yu L, Li T, Robertson Z, Dean J, Gu NF, Feng GY et al. No association between polymorphisms of methylenetetrahydrofolate reductase gene and schizophrenia in both Chinese and Scottish populations. Mol Psychiatry 2004; 9: 1063–1065.
Tan EC, Chong SA, Lim LCC, Chan AOM, Teo YY, Tan CH et al. Genetic analysis of the thermolabile methylenetetrahydrofolate reductase variant in schizophrenia and mood disorders. Psychiatr Genet 2004; 14: 227–231.
Munafò MR, Flint J . Meta-analysis of genetic association studies. Trends Genet 2004; 20: 439–444.
Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 2004; 50: 3–32.
Davey Smith G, Ebrahim S . Mendelian randomization: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003; 32: 1–22.
Botto L, Yang Q . 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 2000; 151: 862–877.
Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM . Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 1998; 55: 1449–1455.
Allain P, Le Bouil A, Cordillet E, Le Quay L, Bagheri H, Montastruc JL . Sulfate and cysteine levels in the plasma of patients with Parkinson's disease. Neurotoxicology 1995; 16: 527–529.
Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T . Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry 1997; 154: 426–428.
Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MWP, Reynolds EH . Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry 2000; 69: 228–232.
Bjelland I, Tell GS, Vollset SE, Refsum H, Ueland PM . Folate, vitamin B12, homocysteine, and the MTHFR 677C>T polymorphism in anxiety and depression. Arch Gen Psychiatry 2003; 60: 618–626.
Lipton SA, Kim WK, Choi YB, Kumar S, D'Emelia DM, Rayudu PV et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA 1997; 94: 5923–5928.
Mattson MP, Shea TB . Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci 2003; 26: 137–146.
Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L et al. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci 2000; 20: 6920–6926.
Ho PI, Ortiz D, Rogers E, Shea TB . Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res 2002; 70: 694–702.
Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J Neurosci 2002; 22: 1752–1762.
Rosenblatt DS . Inherited disorders of folate transport and metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill: New York, 1995, pp 3111–3128.
Homocysteine Lowering Trialists' Collaboration. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ 1998; 316: 894–898.
Godfrey PSA, Toone BK, Carney MWP, Flynn TG, Bottiglieri T, Laundy M et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet 1990; 336: 392–395.
MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991; 38: 131–137.
McGrath JJ, Feron FP, Burne TH, Mackay-Sim A, Eyles DW . The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Ann Med 2003; 35: 86–93.
Acknowledgements
Martin den Heijer is recipient of a VENI-grant from the Netherlands Foundation of Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muntjewerff, J., Kahn, R., Blom, H. et al. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Mol Psychiatry 11, 143–149 (2006). https://doi.org/10.1038/sj.mp.4001746
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001746
Keywords
This article is cited by
-
Studies of the Association of Genetic Polymorphism C677T in the Methylenetetrahydrofolate Reductase Gene with Symptom Severity in Schizophrenia Patients
Neuroscience and Behavioral Physiology (2021)
-
Prevalence and clinical demography of hyperhomocysteinemia in Han Chinese patients with schizophrenia
European Archives of Psychiatry and Clinical Neuroscience (2021)
-
MTHFR Ala222Val polymorphism and clinical characteristics confer susceptibility to suicide attempt in chronic patients with schizophrenia
Scientific Reports (2020)
-
Homocysteine level, body mass index and clinical correlates in Chinese Han patients with schizophrenia
Scientific Reports (2020)
-
Disturbed homocysteine metabolism is associated with cancer
Experimental & Molecular Medicine (2019)