Abstract
Autism is a severe behavioral disorder characterized by pervasive impairments in social interactions, deficits in verbal and nonverbal communication, and stereotyped, repetitive patterns of behaviors and interests. Recently, a new rodent model of autism was created by exposure of rat fetuses to valproic acid (VPA) on the 12.5th day of gestation (VPA rats). The model has striking anatomical, pathological, and etiological similarities to human data; however, it has not been characterized behaviorally. In order to determine if VPA rats present behavioral aberrations observed in autism, their behavior was extensively evaluated in a battery of tests. The results of the present experiments demonstrate that VPA rats exhibit: (1) lower sensitivity to pain and higher sensitivity to nonpainful stimuli, (2) diminished acoustic prepulse inhibition, (3) locomotor and repetitive/stereotypic-like hyperactivity combined with lower exploratory activity, and (4) decreased number of social behaviors and increased latency to social behaviors. In addition, VPA rats showed delayed maturation, lower body weight, delayed motor development, and attenuated integration of a coordinated series of reflexes, delayed nest-seeking response mediated by olfactory system, and normal negative geotaxis. Interestingly, all behavioral aberrations described in this paper appear before puberty, which could distinguish the VPA rat model of autism from other animal models of neurodevelopmental disorders, especially rodent models of schizophrenia. Our results bring further support to validity of the proposed VPA animal model of autism, suggesting similarities between the observed pattern of behavioral alterations in VPA rats and features of disturbed behavior in autistic patients.
Similar content being viewed by others
Introduction
Autism is a severe behavioral disorder that develops in the first 3 years of life. It is characterized by pervasive impairments in social interactions, deficits in verbal and nonverbal communication, and stereotyped, repetitive patterns of behaviors and interests. The observed behavioral disturbances also include self-injury, hyperactivity, and aberrant sensitivity to sensory stimulation. Autism prevalence rate ranges between 1 in 500 to 1 in 2500 (Sugiyama et al, 1992; Bryson and Smith, 1998; California Health and Human Service Agency, 1999). The etiology of autism is not known but it has a strong genetic component (Folstein and Piven, 1991; Bailey et al, 1995; Folstein and Rosen-Sheidley, 2001), and an exposure to at least three teratogens appears to be a risk factor for the disorder: thalidomide (Strömland et al, 1994), valproic acid (VPA) (Christianson et al, 1994; Williams and Hersh, 1997; Williams et al, 2001), and ethanol (Nanson, 1992).
A study of patients in the Swedish thalidomide registry revealed that about 30% of children exposed to thalidomide on the 20–24th day of gestation became autistic, which is dozens of times more than in the general population (Miller and Strömland, 1993; Strömland et al, 1994). The 20–24th day of gestation is the time of neural tube closure and development of the first neurons, which form the motor nuclei of the cranial nerves. Histological, anatomical, and MRI studies of nonthalidomide autistic patients’ brains confirm existence of very early developmental deficits in this disorder and indicate: (1) abnormalities of the cranial nerve nuclei, (2) hypoplasia of brainstem structures, (3) reduced volume of posterior parts of cerebellar vermis and hemispheres with a loss of Purkinje cells, and (4) injury to deep nuclei of the cerebellum (Bauman and Kemper, 1985, 1994, 1997; Gaffney et al, 1988; Hashimoto et al, 1989, 1992, 1995; Kemper and Bauman, 1993; Courchesne, 1994, 1997, 2002; Rodier et al, 1996, 1997). The absence of gliosis following neuron loss in the described structures suggests that the damage could have occurred only during very early stages of brain development (Sumi and Hager, 1968; Gilles, 1983).
Since thalidomide does not have the same teratogenic effect in rodents as in primates (Schumacher et al, 1968), VPA was used to injure rats’ brainstems in utero (Rodier et al, 1996, 1997). Somatic effects of VPA are known to be similar to those of thalidomide (Binkerd et al, 1988), and its teratogenic effect is also observed in human beings (Lindhout et al, 1992; Ardinger et al, 1998) also causing, in addition to somatic dysfunction, CNS dysfunction described as fetal valproate syndrome, which has recently been connected with autism (Christianson et al, 1994; Williams and Hersh, 1997; Williams et al, 2001; Moore et al, 2000; Bescoby Chambers et al, 2001). In rats, the neural tube closes on day 11, and within the 12th day of gestation, production of the motor nuclei of trigeminal, abducens, and hypoglossal nerves is completed (Altman and Bayer, 1980). Malfunctions of the targets of these neurons were reported in thalidomide-induced autism (Strömland et al, 1994). Offspring of female rats injected with VPA on the 12.5th day of gestation show the following brain abnormalities, resembling those found at autopsy and in brain-imaging studies of autistic patients: (1) diminished number of motor neurons in the oculomotor, trigeminal, abducens, and hypoglossus nuclei of cranial nerves; (2) shortening of the region caudal to the facial nucleus and lengthening of the region rostral to the facial nucleus; (3) smaller cerebella with reduction of a number of Purkinje cells both in the hemispheres and in vermis (greater in posterior than in the anterior lobe); and (4) reduced cerebellar nucleus interpositus (corresponding to the globose and emboliform nuclei in humans) (Rodier et al, 1996, 1997; Ingram et al, 2000).
The aim of our study was to investigate behavioral changes in rats exposed to VPA on day 12.5 of gestation, which has been proposed on the basis of the above-described anatomical similarities to human data as an animal model of autism. Therefore, we selected four behavioral patterns corresponding to those frequently observed in autism for examination under laboratory conditions: (1) nociception, because of the reported changes in endogenous opioids systems in a subgroup of autistic patients (Ross et al, 1987; Cazzullo et al, 1999) and their lowered or even abolished sensitivity to pain (Tordjman et al, 1999; Militerni et al, 2000) and hypersensitivity to some nonpainful stimuli (Ritvo et al, 1968; Ayres and Tickle, 1980; Grandin, 1984); (2) sensorimotor gating measured by acoustic prepulse inhibition, as information processing and attention deficits have been consistently reported in autism (Harris et al, 1999; Townsend et al, 2001; Allen and Courchesne, 2001; Ceponiene et al, 2003), and sensorimotor gating is impaired in autism spectrum disorders (McAlonan et al, 2002); (3) locomotor, exploratory, and repetitive/stereotypic activity, as hyperactivity and lower exploration have been described in autistic patients (De Moura-Serra, 1990; Pierce and Courchesne, 2001), and repetitive/stereotyped behaviors belong to the main symptoms of autism (American Psychiatric Association, 1994); and (4) social behaviors, whose diminution is a hallmark feature of autism (American Psychiatric Association, 1994). In addition, we measured postnatal growth, maturation, and behavioral development.
Materials and Methods
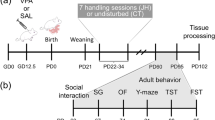
Animals
Female Wistar rats (IP PAS breeding colony), with controlled fertility cycles, were mated overnight, and the morning when spermatozoa were found was designated as the first day of gestation. Females received a single intraperitoneal injection of 600 mg/kg sodium valproate on the 12.5 day after conception, and control females were injected with physiological saline at the same time. Sodium valproate (Sigma) was dissolved in saline at a concentration of 250 mg/ml. Administration of this dose to rats during embryogenesis has been shown to result in the maximum level of total VPA (900 μg/ml) in maternal plasma in less than 1 h, with a mean plasma elimination half-life of 2.3 h (Binkerd et al, 1988). Valproate-treated females and their offspring were healthy and a number of animals per litter were normal compared to controls. Females were housed individually and were allowed to raise their own litters, the litters were not culled, except the litters prepared for the growth and behavioral development studies, which were culled to eight animals per litter (four males, four females) and were excluded from other behavioral tests. The offspring were weaned on postnatal day (PND) 23 and rats of either sex were housed separately. Experiments were carried out on male offspring of the females described above in two developmental periods: prepubertal adolescence (E 30–50, 120–140 g at the beginning of the tests) and adulthood (E 90–120, 280–320 g at the beginning of the tests). Behavioral tests were conducted in the same sequence (nociception and tactile threshold–sensorimotor gating–locomotor and exploratory activity–social behavior) in both developmental periods on groups of 8–12 rats, except social behavior in adulthood tested on groups of five animals. The animals were kept five to a cage (60 × 38 × 20 cm3), with controlled temperature of 21±1°C, and light conditions (lights on at 08:00, lights off at 20:00, reversed in social behavior tests in adulthood). Rats had free access to food (standard laboratory pellets) and water. All the experiments were performed in the light phase between 09:00 and 15:00, except social behavior tests in adult rats, which were performed in the dark phase at the same time. All experiments were carried out according to the NIH Guide for Care and Use of Laboratory Animals and were approved by the Local Bioethics Committee.
Postnatal Growth and Maturation Development
Weight gain was controlled on PNDs 7, 14, 23 and 50, 90, 180.
Eye opening was observed once daily from days 12 to 16 and rated as follows: 0—both eyes closed; 1—one eye open; and 2—both eyes open.
Behavioral Tests and Procedures
Behavioral development
Pups were subjected to behavioral developmental tests according to the procedures described by St Omer et al (1991). All tests were conducted on groups of eight animals (four rats/two litters/group).
Negative geotaxis: This behavior was observed once daily on PNDs 7–10. Pups were timed for completing a 180° turn when placed in a head down position on a 25° inclined surface. Negative geotaxis reflects vestibular function, motor development, and activity (Altman and Sudarshan, 1975).
Swimming performance: An aquarium filled with water (28–29°C) was used for swimming test on PNDs 8, 10, 12, and 16. Each animal was put at the center of the aquarium and was observed for 5–10 s. Swimming performance was evaluated according to the position of nose and head (angle) on the surface of the water. The angle of swimming was rated as follows: 0—head and nose below the surface; 1—nose below the surface; 2—nose and top of head at or above the surface but ears still below the surface; 3—the same as two except that water line was at mid-ear level; and 4—the same as three except that water line was at the bottom of ears. Thereafter, the test pups were dried and returned to the home cages. Swimming is a measure of motor development and integration of coordinated series of reflex responses (Schapiro et al, 1970).
Olfactory discrimination: This test was conducted daily on PNDs 9–11. The apparatus consisted of a plastic container 20 × 8 × 8 cm3 (l × w × h), two small bins, and a clear plastic cover, which was placed over the bins and container. One end of the container contained a bin filled with clean bedding, while at the other end, there was a bin filled with home cage bedding. A 3 cm2 area demarcated the center of the screen. A line was drawn on the screen above each bin. Each pup was placed in the centrally demarcated region on the screen, and the latency to enter the home bedding side by crossing the designed line with the front paws and head was timed. Central placement of the pup was balanced by altering the pup facing to or away the experimenter. Age of home bedding was balanced across the groups and averaged 3 days old at testing. This test reflects a nest-seeking response mediated by the olfactory system (Gregory and Pfaff, 1971).
Nociception and tactile threshold
Nociceptive effects were evaluated using tail flick and thermal paw withdrawal tests. Tactile threshold was determined by von Frey test. The tail flick test was carried out using a tail flick analgesia meter (TF-01, Porfex, Poland). An animal was gently restrained by hand, and radiant heat was directed onto its tail. The cutoff time was 9 s. Tail flick measurements were taken three times at 30 s intervals. The thermal paw withdrawal test was carried out using an Analgesia Meter apparatus (Mod 33, IITC, Inc., USA). Animals were put into single Plexiglas cages. Each hindpaw sole was stimulated by focused light two times at intervals of 2 min. The cutoff time was 12 s. Tactile threshold was assessed using calibrated von Frey filaments (Stoelting, Chicago, IL, USA) presented serially to the hindpaw in ascending order of strength (0.4–26 g), and the mechanical threshold was determined as a rapid withdrawal of the paw immediately after application of the stimulus.
Sensorimotor gating—prepulse inhibition
A startle apparatus (Columbus Instruments, Ohio) consisted of three plastic, transparent cages, equipped with movable platform floor attached to a sensor, which recorded vertical movements of the floor. A loudspeaker was suspended above all cages, and the cages were placed in a soundproof cabinet. A transient force resulting from up-and-down movements of the floor, evoked by a startle reaction to acoustic stimuli, was recorded by PC using a recording window of 200 ms measured from the onset of acoustic stimulus. The amplitude of a startle response was defined as a difference between the average force detected within a recording window and the force measured immediately before the stimulus onset. The threshold was set at 10 g (adolescent males) and 20 g (adult rats), and allowed for correct evaluation of the maximum response in all the animals tested.
The first experiment started with an adaptation period during which the animals were placed in experimental cages for 5 min and exposed to a 68 dB background white noise. Following habituation, each rat was confronted with two types of acoustic stimuli, pulse alone trials in which acoustic stimuli of 120 dB (4000 Hz, duration of 40 ms) were applied, and prepulse+pulse trials in which a tone of 120 dB (4000 Hz, duration of 40 ms) was preceded by a prepulse of 75 dB (4000 Hz, duration of 20 ms) applied 100 ms earlier. Half of each group of animals was treated with a series of 20 pulse stimuli, followed by a series of 20 prepulse+pulse stimuli. The other half of each group received an opposite sequence of acoustic stimuli. Each trial was separated by an interstimulus interval of 20 s. Prepulse inhibition was calculated as the percentage of inhibition of the startle amplitude evoked by pulse alone: ((pulse−prepulse)/pulse) × 100. In the second experiment, additional groups of animals were tested with different prepulse–pulse interval times (30, 100, 300 ms) with a series of 10 pulse stimuli alone and three series of 10 prepulse+pulse stimuli with trials separated by an interstimulus interval of 10 s, applied in a pseudorandom order. Sound parameters and adaptation procedure were the same as in the first experiment.
Locomotor, repetitive/stereotypic-like, and exploratory activity
The locomotor activity of rats was recorded individually for each animal in Opto-Varimex cages (Columbus Instruments, USA), linked on-line to an IBM-PC compatible computer. Each cage (43 × 44 cm2) was equipped with 15 infrared emitters located on the x- and y-axis, and with an equivalent number of receivers on the opposite walls of the cage. The behavior of rats was analyzed using Auto-track software (Columbus Instruments, USA). The locomotor activity was defined as a breakage of three consecutive photobeams. The time of repetitive/stereotypic-like activity was defined as the sum of time intervals (1/10th of a second) in which there was a movement, but an animal did not cross three consecutive photobeams. The animal would have to repeatedly break and make the same three beams for the time interval (1/10th of a second) to be recorded as time of repetitive/stereotypic-like activity. The number of repetitive/stereotypic-like movements was defined as the number of repeated breaks of the same beam in 1/10th of a second. Locomotor and stereotypic-like behavioral patterns were assessed by comparing changes in the mean activity across a 60-min testing session divided into six 10-min intervals.
The exploratory activity was assessed in a small open field. The apparatus consisted of a wooden rectangular box measuring 66 × 57 × 40 cm3 (l × w × h) with two holes in shorter and three in longer walls of the box located regularly in each wall. Number of rearings and hole-pokings (nose of an animal put inside the hole) were measured during a 3-min time session. Background noise was produced by a radio.
Social behavior
The test area for adolescent rats consisted of an acrylic plastic circular cage measuring 38 × 26 cm2 (diameter × height) with approximately 2 cm of wood shavings covering the floor. Adult rats were tested in aquarium measuring 60 × 40 × 40 cm3 (l × w × h) with approximately 2 cm wood shavings covering the floor. The test cage and aquarium were illuminated by a 40 W red light bulb mounted 60 cm above them. Background noise was produced by a radio.
Social (play) behavior: The test was performed under dim light/ unfamiliar conditions, which means that the animals were tested under red light in a novel test cage. On the day of the experiment, the animals were socially isolated in macrolone cages measuring 43 × 28 × 15 cm3 (l × w × h) for 3.5 h prior to the experiment. This isolation period has been shown to produce a half maximal increase in the amount of social play (Niesink and Van Ree, 1989). The test consisted in placing two animals from the same group but different litters and cages (VPA vs VPA, Control (Con) vs Con) into the test cage for 15 min. Pairs were tested in a randomized order for groups and the animals did not differ by more than 15 g in body weight. Animals were tested between 30 and 35 days of life. Behavior was assessed for a pair of animals, so behavior of the individual animal was not analyzed. Latency to pinning (one of the animals is lying with its dorsal surface on the floor of the test cage with the other animal standing over him), total duration, and frequency of pinning were measured. Latency to social behavior unrelated to social play behavior (following or approaching the test partner, mounting or crawling over the test partner, sniffing or grooming any part of the body of the test partner), its total duration and frequency were also measured.
Social behavior in adulthood: At 3 weeks before social behavior was tested, the animals were housed under reversed light conditions (lights off at 08:00, lights on at 20:00). At 1 week before the experiment, rats from both groups were socially isolated. This isolation period has previously been shown to produce a maximal increase in social behavior (Niesink and Van Ree, 1982). The stimulus animals were housed in groups of 5 per cage. All animals were placed individually in the test cage two times daily for 5 min for 2 days prior to the experimentation day in order to reduce the stress due to the novel environment. Animals were tested between days 80 and 90 of life. The test consisted in placing one isolated animal (from VPA or Con group) and one stimulus animal into the test cage for 10 min. Animals were tested in randomized order for groups, and the weight differences between test partners were kept as small as possible. Behavior was assessed for an individual animal (VPA or Con). Latency to social behavior, total duration and frequency of social exploration, and contact were measured, including the following behaviors: sniffing or licking any part of the body of the conspecific except the anogenital area, crawling or mounting (standing on hind legs and putting one or two forpaws on the back of conspecific or climbing over the conspecific), and approaching or following the conspecific. Anogenital investigation (sniffing or licking the anogenital area of the other rat) was measured separately.
Statistics
The results were statistically assessed by Student's t-test for independent samples, and in the case of nonhomogenicity by Kolmogorow–Smirnow and Mann–Whitney U-tests. Weight gain and ontogeny of olfactory discrimination were analyzed by ANOVA with the day of testing as a repeated measure followed by LSD post hoc test. The confidence limit of p<0.05 was considered statistically significant.
Results
Postnatal Growth and Maturation (Figure 1)
Weight gain (Figure 1a)
VPA rats had lower body weight. ANOVA with day of testing as a repeated measure showed a significant effect of the group (F(1,14)=421.7, p<0.001), day of testing (F(5,70)=1405.2, p<0.001), and group × day of testing interaction (F(5,70)=409.1, p<0.005). Post hoc testing revealed lower body weight in VPA group on PNDs 23 (p<0.001), 50 (p<0.05), 90 (p<0.05), and 180 (p<0.05), with no difference on PNDs 7 and 14.
Body weight (a), maturation (b), and behavioral development (c, d) in rats prenatally exposed to VPA. Data expressed as mean±SEM, n=8/2 litters/group. *p<0.05, **p<0.01, ***p<0.001 rats prenatally exposed to VPA (filled bars and squares) compared to control group (open bars and squares) (ANOVA, Mann–Whitney U-test).
Eye opening (Figure 1b)
There was a delay in eye opening in VPA rats on PNDs 13 (U=12, df=14, p<0.04) and 14 (U=6.5, df=14, p<0.04), with no difference on PNDs 12, 15, and 16.
Behavioral Tests
Behavioral development (Figure 1)
Olfactory discrimination ( Figure 1c ): The olfactory discrimination of home bedding odor and nest-seeking behavior mediated by olfactory clues were affected in VPA rats. ANOVA with day of testing as a repeated measure showed a significant effect of the group (F(1,14)=14.51, p<0.002) and group × day interaction (F(2,28)=4.7, p<0.02), and approaching significance difference for day of testing (F(2,28)=2.63, p<0.08). Post hoc testing revealed longer latency to reach home bedding in VPA group on PND 9 (p<0.001), with no difference on PNDs 10 and 11.
Swimming performance ( Figure 1d ): The ontogeny of swimming behavior was significantly delayed in VPA rats on PNDs 8 (U=0, df=14, p<0.001) and 12 (U=8, df=14, p<0.02), with no differences on PNDs 10 and 14.
Negative geotaxis: The ontogeny of negative geotaxis was not significantly altered in VPA rats (data not shown).
Nociception (Figure 2)
Thermal nociceptive thresholds (a, b) and mechanical allodynia (c) in adolescent and adult rats prenatally exposed to VPA. Data expressed as mean±SEM, n=8 for each group. **p<0.01, ***p<0.001 rats prenatally exposed to VPA (filled bars) compared to control (Con, open bars) (Kolmogorow–Smirnow test, Student's t-test).
The nociceptive threshold in VPA rats was increased significantly, both in adolescence (t=−9.1, df=14, p<0.001) and adulthood (KS, df=14, p<0.001), as measured by tail flick test (Figure 2a), and in adulthood (t=−4.81, df=14, p<0.001) but not in adolescence as measured by paw withdrawal test (Figure 2b). Tactile threshold measured by von Frey filaments was lowered in adolescent VPA rats (t=3.55, df=14, p<0.003), with no difference in adulthood (Figure 2c).
Sensorimotor gating—prepulse inhibition (Figure 3)
Acoustic prepulse inhibition in rats prenatally exposed to VPA. Data expressed as mean±SEM, n=12 for each group in adolescence and 10 in adulthood. Data from Experiment 1 (a) and 2 (b) are shown only for 100 ms interval between prepulse and pulse. There was no difference in prepulse inhibition for 30 and 300 ms prepulse–pulse intervals used in Experiment 2. *p<0.05, **p<0.01 rats exposed prenatally to VPA (filled bars) compared to control (Con, open bars) (Student's t-test, Kolmogorow–Smirnow test).
VPA rats demonstrated decreased prepulse inhibition. This effect was observed in the first experiment only in adolescence (t=2.18, df=22, p<0.04) but not adulthood (Figure 3a), and in the second experiment both in adolescence (KS, df=18, p<0.005) and adulthood (t=2.12, df=18, p<0.05), but only for 100 ms prepulse–pulse interval (Figure 3b). Data obtained in the second experiment for 30 and 300 ms prepulse–pulse intervals were not shown. There was no difference in startle amplitude reaction between VPA and control group in either experiments (data not shown).
Locomotor, repetitive/stereotypic-like, and exploratory activity (Figures 4 and 5)
Exploratory behaviors (rearing, hole poking) measured in 3 min test in the small open field in adolescent and adult rats prenatally exposed to VPA. Data expressed as mean±SEM, n=8 for each group. *p<0.05, **p<0.01, ***p<0.001 rats exposed prenatally to VPA (filled bars) compared to control (Con, open bars) (Kolmogorow–Smirnow test, Student's t-test).
Locomotor and repetitive/stereotypic-like behaviors measured across 10-min time blocks in 60 min session in auto track cages in adolescence and adulthood of rats prenatally exposed to VPA. Data are expressed as mean±SEM, n=8 for each group. *p<0.05, **p<0.01, ***p<0.001 rats prenatally exposed to VPA (filled bars) compared to control (open bars) (Kolmogorow–Smirnow test, Student's t-test).
VPA adolescent rats were hyperactive in auto-track cages in 10–20 min interval (t=−4.55, df=14, p<0.001) and demonstrated more time spent on repetitive/stereotypic-like behaviors in 0–10 min (t=−2.45, df=14, p<0.02) and 10–20 min intervals (t=−6.13, df=14, p<0.001), and more repetitive/stereotypic-like behaviors in 10–20 min interval (t=−5.64, df=14, p<0.001), with result approaching significance in 0–10 min interval (p<0.06). Adult VPA rats were hyperactive in 40–50 min interval (t=−3.4, df=14, p<0.004) with longer duration of repetitive/stereotypic-like activity in 40–50 min (t=−4.15, df=14, p<0.001) and 50–60 min intervals (t=−3.84, df=14, p<0.002), and increased number of repetitive/stereotypic-like behaviors in 40–50 min (t=−4.73, df=14, p<0.001) and 50–60 min intervals (t=−2.74, df=14, p<0.02).
Exploratory activity of VPA rats measured in small open field (Figure 5) was lowered compared to control group in respect to both rearing and hole-poking behaviors in adolescence (KS, df=14, p<0.001, and p<0.005, respectively) and adulthood alike (t=2.88, df=14, p<0.01, and t=3.34, df=14, p<0.005, respectively).
Social behavior (Figures 6 and 7)
Number of social explorations and anogenital inspections, total number of social behaviors (a), and latency to social behavior and duration of social behaviors (b) in adult rats prenatally exposed to VPA measured during 10 min test. Data are expressed as mean±SEM, n=5 for each group. *p<0.05 rats prenatally exposed to VPA (filled bars) compared to control (open bars) (Student's t-test).
Number of pinnings (a), latency to and duration of pinning, latency to social nonplay behaviors and duration of social nonplay behaviors (b) measured during 15 min test in adolescent rats prenatally exposed to VPA. Data are expressed as mean±SEM, n=6 pairs/group. **p<0.01 rats prenatally exposed to VPA (filled bars) compared to control (Con, open bars) (Student's t-test).
Social (play) behavior: VPA adolescent rats compared to control group showed a decrease in frequency of pinning (t=3.16, df=10, p<0.01) (Figure 6a). Latency to pinning, duration of pinning, latency to social nonplay behavior, and duration of social nonplay behavior were not changed compared to control (Figure 6b). However, latency to social nonplay behavior in VPA group was longer and short of significant (p<0.07).
Social behavior in adulthood: VPA adult rats compared to control group showed longer latency to social behavior (t=−2.67, df=8, p<0.03) (Figure 7a) and decreased the number of social explorations (t=2.27, df=8, p<0.05) (Figure 7b). There were no significant differences in duration of social behaviors (Figure 7a), sum of social behaviors (social explorations+anogenital inspections), and number of anogenital inspections between VPA and control groups (Figure 7b).
Discussion
The results of the present experiments demonstrate that prenatal exposure to VPA on day 12.5 of gestation has long-term and selective effects on postnatal behaviors in rats. Compared with control group, VPA rats demonstrate: (1) lower sensitivity to pain involving both spinal (tail flick) and supraspinal (paw withdrawal) levels (but the latter was observed only in adulthood), and higher sensitivity to nonpainful stimuli (however, allodynia was observed only in adolescence); (2) diminished acoustic prepulse inhibition; (3) locomotor and repetitive, stereotypic-like hyperactivity combined with lower exploratory activity; (4) decreased number of social behaviors and increased latency to social behaviors. In addition, VPA rats showed delayed maturation (later eye opening), lower body weight, delayed motor development and attenuated integration of coordinated series of reflexes (swimming test), delayed nest-seeking response mediated by olfactory system (olfactory discrimination), and normal negative geotaxis, suggesting normal vestibular function and normal activity.
Most of these behavioral abnormalities have been observed in autistic persons. Indeed, autistic patients express: (1) lowered or even abolished sensitivity to pain (Gillberg and Coleman, 1992; Tordjman et al, 1999; Militerni et al, 2000) and hypersensitivity to some nonpainful stimuli (Ritvo et al, 1968; Ayres and Tickle, 1980; Grandin, 1984, 2) deficits of information processing and attention with impaired sensorimotor gating (Harris et al, 1999; Townsend et al, 2001; Allen and Courchesne, 2001; McAlonan et al, 2002; Ceponiene et al, 2003, 3) hyperactivity with lowered exploratory activity (De Moura-Serra, 1990; Pierce and Courchesne, 2001) and motor repetitive/stereotypic behaviors (Golse, 1986; Sauvage et al, 1993; American Psychiatric Association, 1994; Pierce and Courchesne, 2001), as well as (4) social interaction deficits (American Psychiatric Association, 1994). The maturational and developmental data obtained from VPA rats also stay in concordance with prolonged persistence of some reflexes and delayed appearance of others observed in a subgroup of autistic-to-be infants (Teitelbaum et al, 1998, 2002). The characteristic cluster of disturbances in movement patterns described in autistic-to-be infants may be symptomatic for later delay or even the inability to reach developmental milestones observed in many autistic patients (Bristol-Power and Spinella, 1999; Filipek et al, 1999, 2000).
The behavioral patterns described in our experiments suggest maturational delay and attenuation of social bonding in the early stage of life of VPA rats (maturational and developmental data), disturbed functioning of sensory systems (nociceptive and touch thresholds), and impairment of attention and/or sensorimotor gating (prepulse inhibition). However, decreased prepulse inhibition was observed in adult VPA rats only when several different prepulse–pulse intervals were used (Experiment 2). This may suggest that the deficit may be observable/enhanced with more complex sensory stimulation. This result is consistent with data from other studies. For example, Varty et al (1999) found a more robust prepulse inhibition deficit induced by isolation rearing when using variable prepulse–pulse intervals. Impaired sensorimotor gating may theoretically lead to sensory overload, cognitive fragmentation, and disturbed habituation, thus leading to stronger reactions to environmental stimulation. This can manifest itself as increased repetitive/stereotypic-like behaviors and hyperlocomotion (which may be treated as compulsive non goal-oriented behavior and interpreted as locomotor stereotypy), both were observed in VPA rats. Increased repetitive/stereotypic-like behavior and hyperlocomotion remain in concordance with reduction of exploration measured in a small open field for adolescent VPA rats. Locomotor activity in open field is thought to be the result of a competition between general arousal and exploratory activities; thus, increased non goal-directed locomotor velocity may suppress exploration (Roth and Katz, 1979; Stefanski et al, 1992). However, decreased exploration with normal locomotor activity in adult VPA rats suggests that the effect may be mediated rather by fear-related inhibition of exploratory activity or decreased motivation to explore a novel environment. Another feature observed in VPA rats—attenuated social play behavior (the earliest affiliative form of behavior functioning to facilitate social development)—may result in the decreased ability to express and understand intraspecific communicative signals and to perform social behaviors in adequate sequences and in appropriate contexts in adulthood (for a review see Vanderschuren et al, 1997). This corresponds to our observation of longer latency to social behaviors and decreased number of social explorations with a normal number of anogenital inspections and duration of total social contact time in adult VPA rats. Interestingly, all behavioral aberrations described in this paper appear before puberty, which could distinguish the VPA rat model of autism from other animal models of psychiatric disorders, especially rodent models of schizophrenia.
As mentioned previously, VPA rats also show brain abnormalities resembling those found for autopsy and in brain-imaging studies of autistic patients (Bauman and Kemper, 1985, 1994, 1997; Gaffney et al, 1988; Hashimoto et al, 1989, 1992, 1995; Kemper and Bauman, 1993; Courchesne, 1994, 1997, 2002; Rodier et al, 1996, 1997; Ingram et al, 2000). Therefore, VPA rodent model of autism appears to parallel both anatomical and functional pathology reported in autism and related neurodevelopmental disorders confirming validity of the model and its usefulness for further studies. These include systematic examination of the effects of substances, which could ameliorate autistic-like behaviors.
In summary, this is the first report describing a broad spectrum of behavioral aberrations in rats exposed to VPA on 12.5th day of gestation. Our results bring further support to the validity of the proposed VPA animal model of autism. The results suggest the existence of similarities between the observed pattern of behavioral alterations in VPA rats and features of disturbed behavior in autistic patients. Much additional research will be needed to test a biochemical basis of the behavioral aberrations observed in VPA rats and it would be critical to combine the process of validating the animal model of autism with the process of identifying reliable biochemical measures of human phenomenology. Nonetheless, similarities in behavioral and anatomic pathology in autism and VPA rats suggest the utility of the VPA rodent model of autism for defining common pathways for dysregulation of normal developmental patterns and assessing the time course and sources of vulnerability to that still not curable disorder. The availability of an animal model of autism also opens the door to rigorous evaluation of the effects of environmental manipulations (enriched/impoverished environment) on the behavioral expression of neuropathological deficits present in VPA rats.
References
Allen G, Courchesne E (2001). Attention function and dysfunction in autism. Front Biosci 6: 105–119.
Altman J, Bayer SA (1980). Development of the brain stem in the rat. J Comp Neurol 184: 1–35 37–56, 905–929.
Altman J, Sudarshan K (1975). Postnatal development of locomotion in the laboratory rats. Anim Behav 23: 896–920.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders 4th edn. American Psychiatric Association: Washington, DC. pp 66–71.
Ardinger HH, Atkin JF, Blackston RD, Elsas LJ, Clarren SK, Livingstone S et al (1998). Verification of fetal valproate syndrome phenotype. Am J Med Genet 29: 171–185.
Ayres AJ, Tickle L (1980). Hyper-responsivity to touch and vestibular stimulation as a predictor of responsivity to sensory integrative procedure by autistic children. Am J Occup Ther 34: 375–381.
Bailey A, La Couter A, Gottesman I, Bolton P, Simonoff E, Yuzda E et al (1995). Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25: 63–78.
Bauman M, Kemper TL (1985). Histoanatomic observations of the brain in early infantile autism. Neurology 35: 866–874.
Bauman ML, Kemper TL (1994). Neuroanatomic observation of the brain in autism In: Bauman ML, Kemper TL (eds). The Neurobiology of Autism. John Hopkins University Press: Baltimore, MD. pp 119–145.
Bauman M, Kemper TL (1997). Is autism a progressive disorder? Neurology 48 (Suppl. 3): A285.
Bescoby Chambers N, Forster P, Bates G (2001). Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol 43: 847.
Binkerd PE, Rowland JM, Nan H, Hendricks AG (1988). Evaluation of valproic acid (VPA). Developmental toxicity and pharmatokinetics in Spraque–Dawley rats. Fund Appl Toxicol 11: 485–493.
Bristol-Power MM, Spinella G (1999). Research on screening and diagnosis in autism: a work in progress. J Autism Dev Disord 29: 435–438.
Bryson SE, Smith IM (1998). Epidemiology of autism: prevalence, associated characteristics, and implications for research and service delivery. Ment Retard Dev Dis Res Rev 4: 97–103.
California Health and Human Services Agency (1999). Changes in the population of persons with autism and PDD in California's Developmental Services System: 1987–1998. Raport to the Legislature. Sacramento, CA.
Cazzullo AG, Musetti MC, Musetti L, Bajo S, Sacerdote P, Panerai A (1999). Beta endorphine levels in peripheral blood mononuclear cells and long-term naltrexone treatment in autistic children. Eur Neuropsychopharmacol 9: 361–366.
Ceponiene R, Lepisto T, Shestakova A, Vanhala R, Alku P, Naatanen R et al (2003). Speech-sound-selective auditory impairment in children with autism: they can perceive but do not attend. Proc Natl Acad Sci USA 100: 5567–5572.
Christianson AL, Chesler N, Kromberg JGR (1994). Fetal valproate syndrome: clinical and neurodevelopmental features in two sibling pairs. Dev Med Child Neurol 36: 357–369.
Courchesne E (1997). Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol 7: 269–278.
Courchesne E (2002). Abnormal early brain development in autism. Mol Psychiatry 7 (Suppl. 2): S21–S23.
Courchesne E, Townsend J, Saitoh O (1994). Brain in infantile autism. Neurology 44: 214–228.
De Moura-Serra J (1990). Le diagnostic de I’autisme dans la perspective de la neuropsychiayrie dèveloppementale. In: Messerschmitt P (eds). Clinique des Syndromes Autistiques. Meloine: Paris. pp 34–42.
Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH, Dawson G et al (2000). Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology 55: 468–479.
Filipek PA, Accardo PJ, Baranek GT, Cook EH, Dawson G, Gordon B et al (1999). The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disor 29: 439–484.
Folstein SE, Piven J (1991). Etiology of autism: genetic influences. Pediatrics 87: 767–773.
Folstein SE, Rosen-Sheidley B (2001). Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet 2: 943–955.
Gaffney GR, Kuperman S, Tsai LY, Minchin S (1988). Morphological evidence for brainstem involvement in infantile autism. Biol Psychiatry 24: 578–586.
Gillberg C, Coleman M (1992). The Biology of the Autistic Syndromes 2nd edn. Mac Keith Press: London.
Gilles FH (1983). Neural damage: inconstancy during gestation In Gilles FH, Leviton A, Dooling EC (eds). The Developing Human Brain: Growth and Epidemiologic Neuropathology. J Wright-PSG: Boston. pp 227–243.
Golse B (1986). L’autisme infantile. Pract Méd 42: 7–25.
Grandin T (1984). My experience as an autistic child and review of selected literature. J Ortho Psychiatry 13: 144–174.
Gregory EH, Pfaff DW (1971). Development of olfactory-guided behavior in infant rats. Physiol Behav 6: 573–576.
Harris NS, Courchesne E, Townsend J, Carper RA, Lord C (1999). Neuroanatomic contributions to slowed orienting of attention in children with autism. Brain Res Cogn Brain Res 8: 61–71.
Hashimoto T, Tayama M, Miyazaki M, Sakaruna N, Yoshimoto T, Murakawa K et al (1992). Reduced brainstem size in children with autism. Brain Dev 14: 94–97.
Hashimoto T, Tayama M, Mori K, Fujino K, Miyazaki M, Kuroda Y (1989). Magnetic resonance imaging in autism: preliminary report. Neuropediatrics 20: 142–146.
Hashimoto T, Tayama M, Murakawa K, Yoshimoto T, Miyazaki M, Harada M et al (1995). Development of the brainstem and cerebellum in autistic patients. J Autism Dev Disord 25: 1–18.
Ingram JL, Peckham M, Tisdale B, Rodier PM (2000). Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol 22: 319–324.
Kemper TL, Bauman ML (1993). The contribution of neuropathologic studies to the understanding of autism. Neurol Clin 11: 175–187.
Lindhout D, Ontzigt JG, Cornel MC (1992). Spectrum of neural tube defects in 34 infants prenatally exposed to antiepileptic drugs. Neurology 42: 111–118.
McAlonan GM, Daly E, Kumari V, Critchley HD, Van Amelsvoort T, Suckling J et al (2002). Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain 125: 1594–1606.
Militerni R, Bravaccio C, Falco C, Puglisi-Allegra S, Pascuci T, Fico C (2000). Pain reactivity in children with autistic disorder. J Headache Pain 1: 53–56.
Miller MT, Strömland K (1993). Thalidomide embryopathy: an insight into autism? Teratology 47: 387–388.
Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T et al (2000). A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet 37: 489–497.
Nanson JL (1992). Autism in fetal alcohol syndrome: a report of six cases. Alcohol Clin Exp Res 16: 558–565.
Niesink RJM, Van Ree JM (1982). Short term isolation increases social interactions of male rats: a parametric analysis. Physiol Behav 29: 819–825.
Niesink RJM, Van Ree JM (1989). Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology 28: 411–418.
Pierce K, Courchesne E (2001). Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry 49: 655–664.
Ritvo ER, Ornitz EM, LaFranchi S (1968). Frequency of repetitive behavior in early infantile autism and its variants. Arch Gen Psychiatry 19: 341–347.
Rodier PM, Ingram JL, Tisdale B, Croog VJ (1997). Linking etiologies in humans and animal models: studies of autism. Reprod Toxicol 11: 417–422.
Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J (1996). Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 370: 247–261.
Ross DL, Klykylo WM, Hitzemann R (1987). Reduction of elevated CSF beta endorphin by fenfluramine in infantile autism. Pediatr Neurol 3: 83–86.
Roth KA, Katz RJ (1979). Stress, behavioral arousal, and open field activity: a re-examination of emotionality in rats. Neurosci Biobehav Rev 3: 247–263.
Sauvage D, Hameury L, Darves-Bomoz JM (1993). Nosographie de l’autisme. Discussion critique des classifications actuelles. Presse Méd 22: 1449–1454.
Schapiro S, Salas M, Vinkovich K (1970). Hormonal effects on ontogeny of swimming ability in rats: Assesment of central nervous system development. Science 168: 147–151.
Schumacher HJ, Terapane J, Jordan RL, Wilson JG (1968). The teratogenic activity of a thalidomide analogue, EM 12, in rabbits, rats, and monkeys. Teratology 5: 233–240.
Stefanski R, Palejko W, Kostowski W, Plaznik A (1992). The comparison of benzodiazepines derivatives and serotonergic agonists and antagonists in two animal models of anxiety. Neuropharmacology 31: 1251–1258.
St Omer VEV, Ali SF, Holson RR, Duhart HM, Salzo FM, Slikker W (1991). Behavioral and neurochemical effects of prenatal methylenedioxymethamphetamine (MDMA). Exposure in rats. Neurotoxicol Teratol 13: 13–20.
Strömland K, Nordin V, Miller MT, Akerstrom B, Gillberg C (1994). Autism in thalidomide embryopathy: a population study. Dev Med Child Neurol 36: 351–356.
Sugiyama T, Takei Y, Abe T (1992). The prevalence of autism in Nagaya, Japan In Naruse H, Ornitz M (eds). Neurobiology of Infantile Autism. Excerpta Medica: Amsterdam. pp 181–184.
Sumi SM, Hager H (1968). Electron microscopic study of the reaction of the newborn rat brain to injury. Acta Neuropathol Berl 10: 324–335.
Teitelbaum P, Teitelbaum O, Fryman J, Maurer R (2002). Infantile reflexes gone astray in autism. J Dev Learn Disord 6: 15–22.
Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer R (1998). Movement analysis in infancy may be useful for early diagnosis of autism. Proc Natl Acad Sci USA 95: 13982–13987.
Tordjman S, Antoine C, Gauvain-Piguard A, Carlier M, Roubertoux P, Ferrari P (1999). Etude des conduits autoagressives, de la reactivite a la douleur et de leurs interrelations chez les enfants autistes. Encephale 25: 122–134.
Townsend J, Westerfield M, Leaver E, Makeig S, Jung T, Pierce K et al (2001). Event-related brain response abnormalities in autism: evidence for impaired cerebello-frontal spatial attention networks. Brain Res Cogn Brain Res 11: 127–145.
Vanderschuren LJ, Niesink RJ, Van Ree JM (1997). The neurobiology of social play behavior in rats. Neurosci Biobehav Rev 21: 309–326.
Varty GB, Marsden CA, Higgins GA (1999). Reduced synaptophysin immunoreactivity in the dentate gyrus of prepulse inhibition-impaired isolation-reared rats. Brain Res 824: 197–203.
Williams G, King J, Cunningham M, Steohan M, Kerr B, Hersh JH (2001). Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol 43: 202–206.
Williams PG, Hersh JH (1997). A male with fetal valproate syndrome and autism. Dev Med Child Neurol 36: 632–634.
Acknowledgements
This research was supported by statutory funds from the State Committee for Scientific Research, Warsaw, Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schneider, T., Przewłocki, R. Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Animal Model of Autism. Neuropsychopharmacol 30, 80–89 (2005). https://doi.org/10.1038/sj.npp.1300518
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300518
Keywords
This article is cited by
-
Degraded inferior colliculus responses to complex sounds in prenatally exposed VPA rats
Journal of Neurodevelopmental Disorders (2024)
-
Dysregulation of the Wnt/β-catenin signaling pathway via Rnf146 upregulation in a VPA-induced mouse model of autism spectrum disorder
Experimental & Molecular Medicine (2023)
-
Saffron and crocin ameliorate prenatal valproic acid-induced autistic-like behaviors and brain oxidative stress in the male offspring rats
Metabolic Brain Disease (2023)
-
Neuroprotective Efficacy of Fisetin Against VPA-Induced Autistic Neurobehavioral Alterations by Targeting Dysregulated Redox Homeostasis
Journal of Molecular Neuroscience (2023)
-
Effect of WiFi signal exposure in utero and early life on neurodevelopment and behaviors of rats
Environmental Science and Pollution Research (2023)