-
PDF
- Split View
-
Views
-
Cite
Cite
Killian A. Welch, Alan Carson, Stephen M. Lawrie, Brain Structure in Adolescents and Young Adults with Alcohol Problems: Systematic Review of Imaging Studies, Alcohol and Alcoholism, Volume 48, Issue 4, July/August 2013, Pages 433–444, https://doi.org/10.1093/alcalc/agt037
Close - Share Icon Share
Abstract
Aims: Alcohol-dependent people who are middle-aged or older have a widespread loss of cortical grey and white matter, particularly in the prefrontal cortex (PFC). We examine if brain abnormalities are detectable in alcohol use disorders before the fifth decade (i.e. <40), and the brain structural differences associated with alcohol abuse/dependence in adolescence. Methods: Case–control studies comparing brain structure in alcohol-abusing/-dependent individuals with normal controls in which the mean age of participants was <40 were identified using Medline, EMBASE and PsychInfo. Studies in which mean age was over and under 21 were considered separately. Results: Twelve papers fulfilled inclusion criteria, five in the adolescent (14–21) and seven in the young adult age range. Two independent groups reported hippocampal and prefrontal volume reductions in adolescents, although this was consistently observed only in females. In young adults (aged 21–40), there were grey matter deficits in the PFC in both sexes. Adult women appeared to, particularly, exhibit white matter differences, evident as reduced area of the corpus callosum. Hippocampal volume reduction was observed in one study of young adults study but not another. Conclusion: The available data suggest that quantitative structural abnormalities of the brain are detectable in young alcohol abusers. There is overlap between the abnormalities seen in adolescents and young adults, although hippocampal volume loss is most consistently seen in the former group. The adolescent hippocampus may be particularly susceptible to alcohol, potentially because of an interaction between adolescent brain development and alcohol exposure.
INTRODUCTION
In their 2005 narrative review, Sullivan and Pfefferbaum (2005) report magnetic resonance imaging (MRI) of ‘uncomplicated’ alcoholics (i.e. those free of the severe syndromes arising from alcohol-associated nutritional deficiencies or electrolytic imbalance) as demonstrating widespread loss of cortical grey and white matter, greatest in the prefrontal cortex (PFC) and white matter. Subsequent reviews report similar findings (Bühler and Mann, 2011). Diffusion tensor imaging (DTI) studies in alcoholic adults demonstrate white matter tract disruption in various brain regions (Pfefferbaum et al., 2000, 2009). The mean age of individuals included in these studies was, however, almost exclusively >40 years, with some individuals in their 70s.
It is less clear whether brain structural abnormalities are detectable in younger alcohol abusers. Several countries experienced substantial increases in alcohol consumption and physical sequelae such as liver disease in young people in recent years (Leon and McCambridge, 2006). As has been suggested for cannabis (Meier et al., 2012), an interaction between adolescent brain development and alcohol consumption is possible. Brain maturational changes, predominantly dendritic arborization, synaptic pruning and myelination, continue throughout adolescence and into early adulthood (Crews et al., 2007), potentially, rendering adolescents particularly vulnerable to the brain structural consequences of alcohol use. Indeed, animal studies suggest that adolescent animals are more susceptible to the acute effects of alcohol, and that the consequences of alcohol exposure persist into adulthood. Illustrating the former, alcohol has a greater impact on n-methyl-d-aspartate receptor-mediated long-term potentiation and spatial memory acquisition in adolescent animals (Swartzwelder et al., 1995; Markwiese et al., 1998), and binge exposure only led to brain damage in adolescent animals (Crews et al., 2000). That the effects of adolescent alcohol exposure can persist into adulthood is demonstrated by abnormalities of neurophysiological function (Slawecki et al., 2001), cognitive abilities (Pascual et al., 2007), risk preference (Nasrallah et al., 2009) and reduced basal forebrain volume (Coleman et al., 2011) in adult animals exposed to alcohol in peri-adolescence. In humans, such an interaction could render individuals with considerable adolescent alcohol exposure at increased risk of neurodevelopmental conditions. In keeping with this possibility, a follow-up study of adolescent users of alcohol and other substances demonstrated that these behaviours, whether or not they persisted into adulthood, predicted a relative decline in visuospatial construction performance over the subsequent 10 years (Hanson et al., 2011).
It is, therefore, important to review structural imaging studies in younger alcohol abusers and to evaluate the (potentially differing) effects in adolescents and young adults separately. We hypothesized that adolescents would exhibit greater effects of alcohol on brain structure than adults, this reflecting disruption of ongoing neurodevelopment. Focusing on younger adults, in general, has the clear benefit of examining the effects of alcohol on the brain in the absence of confounders, such as cerebrovascular disease. This is crucial given that an interaction between age and alcohol consumption on brain structural abnormalities has been reported (Pfefferbaum et al., 1992). An immediate question, however, is what age constitutes youth? Given that alcohol-related brain changes are clearly detectable in ‘uncomplicated’ alcoholics by the fifth decade of life (Pfefferbaum et al., 1992), the cut-off age we took was 40 years. As brain development continues into the early 20s, ‘adolescents’ have been defined here as aged 14–21 years.
MATERIALS AND METHODS
Computerized literature searches were performed on Medline (1980–2013), EMBASE (1980–2013) and PsychInfo (1980–2013). Searches were limited by age, the range chosen including 14–40. The following search terms were used: alcohol abuse OR alcohol dependence OR alcoholism OR alcohol drinking AND neuroimaging OR brain imaging OR MRI OR DTI. Both free-text and expanded medical subject headings were used. Subject headings were adapted to specific subject headings of each database. The search strategy was supplemented by inspecting the reference lists of included articles.
We were primarily interested in case-controlled studies. Studies had to utilize structural MRI or DTI. Rather than looking at regional volume or density DTI examines water diffusion, a higher fractional anisotropy (FA) being assumed to indicate greater white matter integrity. Subjects must have met diagnostic criteria for either alcohol abuse or dependence [subsequently, referred to as ‘alcohol use disorders’ (AUDs)]. Studies focussing on ‘binge drinking’ rather than a defined AUD were excluded. As in the narrative review of Sullivan and Pfefferbaum (2005), studies must have excluded individuals with severe syndromes arising from alcohol-associated nutritional deficiencies or electrolytic imbalance. Studies employing any generally accepted, quantitative image analysis technique directly comparing tissue volume, density or integrity were acceptable. To establish if adolescents are particularly susceptible to alcohol-related brain structural abnormalities, study results were considered in two age groupings; an ‘adolescent’ grouping of studies in which mean age was between 14 and 21, and a ‘young adult’ grouping of mean age 21–40. Though we considered further subdividing groupings into adolescents (14–18), emerging adults (19–25) and adults (25–40), the absence of studies confined to the middle age group meant this was not possible.
Primary research studies were considered for inclusion if peer-reviewed articles in English compared a sample of adolescents/young people (aged 14–40) with an AUD (abuse or dependence) with healthy controls. Ideally, both alcohol problem and control groups would not have abused other drugs. Given the low yield of studies, however, reports were considered if alcohol was the principle drug used and they did not meet the criteria for abuse/dependence on other substances. Studies could compare the groups cross-sectionally, or compare changes over time. If both models of analysis were included in a single study, both analyses are reported. Additionally, if different studies used the same patient sample but investigated different brain structures, then both reports were included. In the case of duplicate publications from the same subject group focussing on the same structures, the one with the largest number of participants was included. To facilitate the comparison of adolescent and adult effects, we have sought to include effect sizes. When not originally published but sufficient data have been provided, we have calculated Cohen's d by the methodology of Thalheimer and Cook (2002).
RESULTS
General results for the systematic review
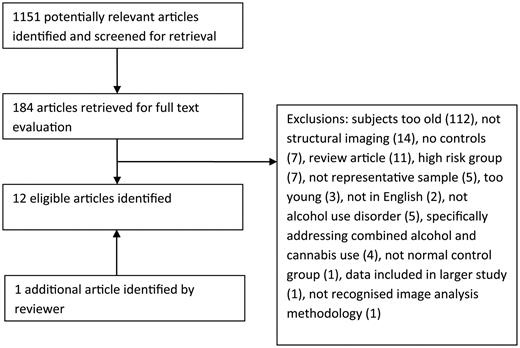
The search identified 1151 study abstracts, which were assessed for inclusion. One hundred and 84 articles were retrieved in full text. Study flow and reasons for exclusion are summarized in Fig. 1. Twelve studies fulfilled inclusion criteria, one of which was identified by a reviewer having been missed by our search methodology. All were cross-sectional. Adolescent and adult studies will be discussed separately.
Results of adolescent systematic review
The five studies examining subjects, with mean age of 14–21, are summarized in Table 1. Only two groups published the case–control studies of adolescents with AUDs eligible for inclusion. These are San Diego-based Tapert et al. and De Bellis et al. in Duke University. The two papers from the San Diego group utilized the same AUD and control groups, focussing on different brain regions. Efforts were made to select adolescents free of potential confounders, such as other substance use and comorbid psychiatric conditions, though a third of cases met criteria for conduct disorder and cases used significantly more cannabis than controls. Importantly, there was no significant difference in the family history of substance use disorder between the groups. The primary finding from the Nagel et al. (2005) study was that adolescents with AUD had significantly smaller left hippocampi than healthy teens. This was significant even after excluding teens with comorbid conduct disorders (P < 0.05, d = 0.70). Interestingly, the hippocampal volume was not significantly related to alcohol-consumption levels. The Medina et al. (2008) study compared both overall PFC and white matter PFC volumes between the groups using the same sample. After controlling for conduct disorder, gender and intracranial volume, AUD adolescents demonstrated non-significantly smaller anterior ventral PFC volumes (P = 0.09). On comparing gender sub-groups, however, females with AUD demonstrated reduced PFC volumes, while males with AUD had larger PFC volumes. The same pattern was observed for PFC white matter volumes. These effects were driven by differences in both anterior ventral and dorsal PFC regions. The family history of substance use was not associated with PFC volume, and the relationships between alcohol use group and PFC volumes remained significant after this was controlled for. There were no significant correlations between lifetime alcohol use, age of onset of regular alcohol use, alcohol withdrawal symptoms or alcohol-dependence symptoms and PFC volume.
Studies investigating structural imaging abnormalities in adolescent problem drinkers
| Study . | Imaging modality . | Image analysis . | Gender AUD/Cont. (M:F) . | Mean age (SD) AUD/Cont. (M/F)a . | Inclusion criteria . | Drinking status . | Estimate of alcohol quantities consumed . | Mean age of onset (SD) of alcohol use/AUD . | Use of other subs . | Structures compared . | Findings . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| De Bellis et al. (2000) | MRI | Semi- automated ROI | 5:7/10:14 | 17.2 (2.2)/17.0 (2.4) Range: 13.5–21.0 | Substance misuse diagnoses made using modified version of DSM-IV Seven lifetime alcohol dependence, five lifetime alcohol abuse. Majority comorbid Axis I disorders, most commonly depression, conduct disorder or PTSD Excluded if medically significant medical or neurological illness or learning disability | No use for 2 weeks prior to scan | No data | No data | Majority (9) history of cannabis abuse or dependence. Smaller numbers had used other substances. Tobacco use not detailed | Total grey and white matter volumes Amygdala, hippocampal and lateral ventricular volumes. Corpus callosum area | Reduced L, R and combined hippocampal volumes (F = 7.06, P = 0.01, d = 0.97; F = 5.04, P = 0.03, d = 0.82; and F = 6.47, P < 0.02, d = 0.93, respectively) Difference not significant when five subjects with PTSD excluded from comparison (F = 4.18, P < 0.06, d = 0.91). No difference total grey and white matter volumes, amygdalar volumes or corpus callosal area |
| De Bellis et al. (2005) | MRI | Semi- automated ROI | 8:6/16:12 | 17.0 (2.1)/16.9 (2.3) Range: 13.5–21.0 | DSM-IV alcohol abuse or dependence. Majority had Axis I comorbidity, most commonly depression or conduct disorder Excluded if medically significant medical or neurological illness or learning disability | No alcohol use in 12 h preceding scan | Mean number of drinks per maximum drinking episode 12.1 ± 0.86 | Age onset AUD: 15.6 (2.4) | Majority of AUD group (11) also had history of cannabis use disorder. Small numbers had used other substances, but not within 2 weeks of scan. Tobacco use not detailed | Total cerebral volume, total PFC volume, grey and white matter PFC volume, volume of thalamus, brainstem, right and left cerebellum | Reduced total PFC (t = −2.38, P = 0.02, d = 0.80) and PFC white matter volumes (t = −2.38, P < .007, d = 0.96) Findings persisted on controlling for cerebral volume but not comorbidity. When both controlled for d = 0.55 and 0.52 for total PFC and PFC white matter, respectively |
| Nagel et al. (2005) | MRI | Semi- automated ROI | 9:5/5:7 | 16.8 (0.7)/16.5 (0.9) Range: 15.2–17.9 | Met DSM-IV criteria for alcohol abuse or dependence. Only additional DSM-IV diagnosis mild-to-moderate conduct disorder (present in 5) Excluded if learning disability, serious medical or neurological problems, significant head injury or psychotropic medication | Abstinent from the use of alcohol or other drugs for 5 days | Mean drinks/month in last 3 months: 43.00 (31.89) | Regular drinking began 14.9 (1.1) years | AUD group did not differ from controls in the family history of substance use disorder, lifetime use of other drugs, recent marijuana use or marijuana abuse/dependence. Significant greater lifetime episodes of marijuana use and cigarettes in the last month | Whole brain grey and white matter volumes Hippocampal volumes | Reduced left hippocampal volume (t = 2.90, P < 0.01, d = 1.08), no reduction in right hippocampal volume (t = 0.51, P = 0.61, d = 0.19) Remained significant on excluding comorbid conduct disorder (t = 1.71, P < 0.05, d = 0.70) |
| De Bellis et al. (2008) | MRI DTI | Voxel-based ROI | 25:7/17:11 | 16.9 (1.2)/15.9 (1.1)b Range: 13.3–19.3 | Recruited from treatment centres. Met DSM-IV criteria for lifetime alcohol dependence or current alcohol abuse. Majority had Axis I comorbidity, most commonly oppositional defiant disorder, depression, attention deficit hyperactivity disorder or conduct disorder Excluded if medically significant medical or neurological illness or learning disability | Mean (SD) number of days since last consumed alcohol 63.7 (88.2) | Mean number of drinks per maximum drinking episode 13.2 ± 8.6 | Regular drinking began 15.6 (1.3) years AUD onset: 14.7 (1.3) years | Majority (24) of AUD group also had history of cannabis use disorder and had smoked cigarettes. Small numbers had used other substances | Corpus callosum divided into seven regions: rostrum, genu, rostral body, anterior mid-body, posterior mid-body, isthmus and splenium. Microstructural integrity of each region compared | FA values in the corpus callosum rostral body higher in the AUD group than in the control group, though not significant after adjusting for age and sex (F = 2.9, P = 0.09, d = 0.4) FA values also higher in the isthmus in the AUD group, which was significant after controlling for age and sex (d = 0.9) |
| Medina et al. (2008) | MRI | Semi- automated ROI | 9:5/5:7 | M: 16.6 (0.7)/16.6 (0.7) F: 17.1 (0.6)/16.5 (1.0) Range: 15.2–17.9 | Met DSM-IV criteria for alcohol abuse or dependence. Only additional DSM-IV diagnosis mild-to-moderate conduct disorder (present in 5) Excluded if learning disability, serious medical or neurological problems, significant head injury or psychotropic medication | Abstinent from the use of alcohol or other drugs for 5 days | Mean drinks/month in last 3 months: 43.00 (31.89) | Regular drinking began 14.9 (1.1) years | AUD group did not differ from controls in the family history of substance use disorder, lifetime use of other drugs, recent marijuana use or marijuana abuse/dependence criteria. Significantly, greater lifetime episodes of marijuana use and use of cigarettes in the last month | PFC volume. Total and divided into following regions: posterior, anterior dorsal and anterior ventral. Also, white matter volume divided into same subregions | Whole group comparison: no significant difference in PFC volume (whole or white matter) between groups. On comparing gender sub-groups, however, females with AUD demonstrated smaller PFC volumes, while males with AUD had larger PFC volumes. The same pattern was observed for PFC white matter volumes Significant group-by-gender interaction for total PFC volume (F = 10.63, P < 0.003, 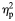 = 0.29), and PFC white matter volume (F = 5.64, P < 0.03, = 0.29), and PFC white matter volume (F = 5.64, P < 0.03, 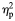 = 0.17). = 0.17). |
| Study . | Imaging modality . | Image analysis . | Gender AUD/Cont. (M:F) . | Mean age (SD) AUD/Cont. (M/F)a . | Inclusion criteria . | Drinking status . | Estimate of alcohol quantities consumed . | Mean age of onset (SD) of alcohol use/AUD . | Use of other subs . | Structures compared . | Findings . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| De Bellis et al. (2000) | MRI | Semi- automated ROI | 5:7/10:14 | 17.2 (2.2)/17.0 (2.4) Range: 13.5–21.0 | Substance misuse diagnoses made using modified version of DSM-IV Seven lifetime alcohol dependence, five lifetime alcohol abuse. Majority comorbid Axis I disorders, most commonly depression, conduct disorder or PTSD Excluded if medically significant medical or neurological illness or learning disability | No use for 2 weeks prior to scan | No data | No data | Majority (9) history of cannabis abuse or dependence. Smaller numbers had used other substances. Tobacco use not detailed | Total grey and white matter volumes Amygdala, hippocampal and lateral ventricular volumes. Corpus callosum area | Reduced L, R and combined hippocampal volumes (F = 7.06, P = 0.01, d = 0.97; F = 5.04, P = 0.03, d = 0.82; and F = 6.47, P < 0.02, d = 0.93, respectively) Difference not significant when five subjects with PTSD excluded from comparison (F = 4.18, P < 0.06, d = 0.91). No difference total grey and white matter volumes, amygdalar volumes or corpus callosal area |
| De Bellis et al. (2005) | MRI | Semi- automated ROI | 8:6/16:12 | 17.0 (2.1)/16.9 (2.3) Range: 13.5–21.0 | DSM-IV alcohol abuse or dependence. Majority had Axis I comorbidity, most commonly depression or conduct disorder Excluded if medically significant medical or neurological illness or learning disability | No alcohol use in 12 h preceding scan | Mean number of drinks per maximum drinking episode 12.1 ± 0.86 | Age onset AUD: 15.6 (2.4) | Majority of AUD group (11) also had history of cannabis use disorder. Small numbers had used other substances, but not within 2 weeks of scan. Tobacco use not detailed | Total cerebral volume, total PFC volume, grey and white matter PFC volume, volume of thalamus, brainstem, right and left cerebellum | Reduced total PFC (t = −2.38, P = 0.02, d = 0.80) and PFC white matter volumes (t = −2.38, P < .007, d = 0.96) Findings persisted on controlling for cerebral volume but not comorbidity. When both controlled for d = 0.55 and 0.52 for total PFC and PFC white matter, respectively |
| Nagel et al. (2005) | MRI | Semi- automated ROI | 9:5/5:7 | 16.8 (0.7)/16.5 (0.9) Range: 15.2–17.9 | Met DSM-IV criteria for alcohol abuse or dependence. Only additional DSM-IV diagnosis mild-to-moderate conduct disorder (present in 5) Excluded if learning disability, serious medical or neurological problems, significant head injury or psychotropic medication | Abstinent from the use of alcohol or other drugs for 5 days | Mean drinks/month in last 3 months: 43.00 (31.89) | Regular drinking began 14.9 (1.1) years | AUD group did not differ from controls in the family history of substance use disorder, lifetime use of other drugs, recent marijuana use or marijuana abuse/dependence. Significant greater lifetime episodes of marijuana use and cigarettes in the last month | Whole brain grey and white matter volumes Hippocampal volumes | Reduced left hippocampal volume (t = 2.90, P < 0.01, d = 1.08), no reduction in right hippocampal volume (t = 0.51, P = 0.61, d = 0.19) Remained significant on excluding comorbid conduct disorder (t = 1.71, P < 0.05, d = 0.70) |
| De Bellis et al. (2008) | MRI DTI | Voxel-based ROI | 25:7/17:11 | 16.9 (1.2)/15.9 (1.1)b Range: 13.3–19.3 | Recruited from treatment centres. Met DSM-IV criteria for lifetime alcohol dependence or current alcohol abuse. Majority had Axis I comorbidity, most commonly oppositional defiant disorder, depression, attention deficit hyperactivity disorder or conduct disorder Excluded if medically significant medical or neurological illness or learning disability | Mean (SD) number of days since last consumed alcohol 63.7 (88.2) | Mean number of drinks per maximum drinking episode 13.2 ± 8.6 | Regular drinking began 15.6 (1.3) years AUD onset: 14.7 (1.3) years | Majority (24) of AUD group also had history of cannabis use disorder and had smoked cigarettes. Small numbers had used other substances | Corpus callosum divided into seven regions: rostrum, genu, rostral body, anterior mid-body, posterior mid-body, isthmus and splenium. Microstructural integrity of each region compared | FA values in the corpus callosum rostral body higher in the AUD group than in the control group, though not significant after adjusting for age and sex (F = 2.9, P = 0.09, d = 0.4) FA values also higher in the isthmus in the AUD group, which was significant after controlling for age and sex (d = 0.9) |
| Medina et al. (2008) | MRI | Semi- automated ROI | 9:5/5:7 | M: 16.6 (0.7)/16.6 (0.7) F: 17.1 (0.6)/16.5 (1.0) Range: 15.2–17.9 | Met DSM-IV criteria for alcohol abuse or dependence. Only additional DSM-IV diagnosis mild-to-moderate conduct disorder (present in 5) Excluded if learning disability, serious medical or neurological problems, significant head injury or psychotropic medication | Abstinent from the use of alcohol or other drugs for 5 days | Mean drinks/month in last 3 months: 43.00 (31.89) | Regular drinking began 14.9 (1.1) years | AUD group did not differ from controls in the family history of substance use disorder, lifetime use of other drugs, recent marijuana use or marijuana abuse/dependence criteria. Significantly, greater lifetime episodes of marijuana use and use of cigarettes in the last month | PFC volume. Total and divided into following regions: posterior, anterior dorsal and anterior ventral. Also, white matter volume divided into same subregions | Whole group comparison: no significant difference in PFC volume (whole or white matter) between groups. On comparing gender sub-groups, however, females with AUD demonstrated smaller PFC volumes, while males with AUD had larger PFC volumes. The same pattern was observed for PFC white matter volumes Significant group-by-gender interaction for total PFC volume (F = 10.63, P < 0.003, 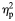 = 0.29), and PFC white matter volume (F = 5.64, P < 0.03, = 0.29), and PFC white matter volume (F = 5.64, P < 0.03, 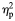 = 0.17). = 0.17). |
Intracranial volume was adjusted for in all volumetric analyses except where otherwise specified.
Effect sizes reported as Cohen's d aside unless otherwise stated. 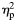 = partial eta squared.
= partial eta squared.
AUD, alcohol use disorder; encompasses either alcohol abuse or dependence; AD, alcohol dependence.
aIf genders have been analysed separately, mean age is displayed separately for each gender. Otherwise mean age is combined.
bSignificant difference between groups.
Studies investigating structural imaging abnormalities in adolescent problem drinkers
| Study . | Imaging modality . | Image analysis . | Gender AUD/Cont. (M:F) . | Mean age (SD) AUD/Cont. (M/F)a . | Inclusion criteria . | Drinking status . | Estimate of alcohol quantities consumed . | Mean age of onset (SD) of alcohol use/AUD . | Use of other subs . | Structures compared . | Findings . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| De Bellis et al. (2000) | MRI | Semi- automated ROI | 5:7/10:14 | 17.2 (2.2)/17.0 (2.4) Range: 13.5–21.0 | Substance misuse diagnoses made using modified version of DSM-IV Seven lifetime alcohol dependence, five lifetime alcohol abuse. Majority comorbid Axis I disorders, most commonly depression, conduct disorder or PTSD Excluded if medically significant medical or neurological illness or learning disability | No use for 2 weeks prior to scan | No data | No data | Majority (9) history of cannabis abuse or dependence. Smaller numbers had used other substances. Tobacco use not detailed | Total grey and white matter volumes Amygdala, hippocampal and lateral ventricular volumes. Corpus callosum area | Reduced L, R and combined hippocampal volumes (F = 7.06, P = 0.01, d = 0.97; F = 5.04, P = 0.03, d = 0.82; and F = 6.47, P < 0.02, d = 0.93, respectively) Difference not significant when five subjects with PTSD excluded from comparison (F = 4.18, P < 0.06, d = 0.91). No difference total grey and white matter volumes, amygdalar volumes or corpus callosal area |
| De Bellis et al. (2005) | MRI | Semi- automated ROI | 8:6/16:12 | 17.0 (2.1)/16.9 (2.3) Range: 13.5–21.0 | DSM-IV alcohol abuse or dependence. Majority had Axis I comorbidity, most commonly depression or conduct disorder Excluded if medically significant medical or neurological illness or learning disability | No alcohol use in 12 h preceding scan | Mean number of drinks per maximum drinking episode 12.1 ± 0.86 | Age onset AUD: 15.6 (2.4) | Majority of AUD group (11) also had history of cannabis use disorder. Small numbers had used other substances, but not within 2 weeks of scan. Tobacco use not detailed | Total cerebral volume, total PFC volume, grey and white matter PFC volume, volume of thalamus, brainstem, right and left cerebellum | Reduced total PFC (t = −2.38, P = 0.02, d = 0.80) and PFC white matter volumes (t = −2.38, P < .007, d = 0.96) Findings persisted on controlling for cerebral volume but not comorbidity. When both controlled for d = 0.55 and 0.52 for total PFC and PFC white matter, respectively |
| Nagel et al. (2005) | MRI | Semi- automated ROI | 9:5/5:7 | 16.8 (0.7)/16.5 (0.9) Range: 15.2–17.9 | Met DSM-IV criteria for alcohol abuse or dependence. Only additional DSM-IV diagnosis mild-to-moderate conduct disorder (present in 5) Excluded if learning disability, serious medical or neurological problems, significant head injury or psychotropic medication | Abstinent from the use of alcohol or other drugs for 5 days | Mean drinks/month in last 3 months: 43.00 (31.89) | Regular drinking began 14.9 (1.1) years | AUD group did not differ from controls in the family history of substance use disorder, lifetime use of other drugs, recent marijuana use or marijuana abuse/dependence. Significant greater lifetime episodes of marijuana use and cigarettes in the last month | Whole brain grey and white matter volumes Hippocampal volumes | Reduced left hippocampal volume (t = 2.90, P < 0.01, d = 1.08), no reduction in right hippocampal volume (t = 0.51, P = 0.61, d = 0.19) Remained significant on excluding comorbid conduct disorder (t = 1.71, P < 0.05, d = 0.70) |
| De Bellis et al. (2008) | MRI DTI | Voxel-based ROI | 25:7/17:11 | 16.9 (1.2)/15.9 (1.1)b Range: 13.3–19.3 | Recruited from treatment centres. Met DSM-IV criteria for lifetime alcohol dependence or current alcohol abuse. Majority had Axis I comorbidity, most commonly oppositional defiant disorder, depression, attention deficit hyperactivity disorder or conduct disorder Excluded if medically significant medical or neurological illness or learning disability | Mean (SD) number of days since last consumed alcohol 63.7 (88.2) | Mean number of drinks per maximum drinking episode 13.2 ± 8.6 | Regular drinking began 15.6 (1.3) years AUD onset: 14.7 (1.3) years | Majority (24) of AUD group also had history of cannabis use disorder and had smoked cigarettes. Small numbers had used other substances | Corpus callosum divided into seven regions: rostrum, genu, rostral body, anterior mid-body, posterior mid-body, isthmus and splenium. Microstructural integrity of each region compared | FA values in the corpus callosum rostral body higher in the AUD group than in the control group, though not significant after adjusting for age and sex (F = 2.9, P = 0.09, d = 0.4) FA values also higher in the isthmus in the AUD group, which was significant after controlling for age and sex (d = 0.9) |
| Medina et al. (2008) | MRI | Semi- automated ROI | 9:5/5:7 | M: 16.6 (0.7)/16.6 (0.7) F: 17.1 (0.6)/16.5 (1.0) Range: 15.2–17.9 | Met DSM-IV criteria for alcohol abuse or dependence. Only additional DSM-IV diagnosis mild-to-moderate conduct disorder (present in 5) Excluded if learning disability, serious medical or neurological problems, significant head injury or psychotropic medication | Abstinent from the use of alcohol or other drugs for 5 days | Mean drinks/month in last 3 months: 43.00 (31.89) | Regular drinking began 14.9 (1.1) years | AUD group did not differ from controls in the family history of substance use disorder, lifetime use of other drugs, recent marijuana use or marijuana abuse/dependence criteria. Significantly, greater lifetime episodes of marijuana use and use of cigarettes in the last month | PFC volume. Total and divided into following regions: posterior, anterior dorsal and anterior ventral. Also, white matter volume divided into same subregions | Whole group comparison: no significant difference in PFC volume (whole or white matter) between groups. On comparing gender sub-groups, however, females with AUD demonstrated smaller PFC volumes, while males with AUD had larger PFC volumes. The same pattern was observed for PFC white matter volumes Significant group-by-gender interaction for total PFC volume (F = 10.63, P < 0.003, 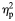 = 0.29), and PFC white matter volume (F = 5.64, P < 0.03, = 0.29), and PFC white matter volume (F = 5.64, P < 0.03, 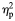 = 0.17). = 0.17). |
| Study . | Imaging modality . | Image analysis . | Gender AUD/Cont. (M:F) . | Mean age (SD) AUD/Cont. (M/F)a . | Inclusion criteria . | Drinking status . | Estimate of alcohol quantities consumed . | Mean age of onset (SD) of alcohol use/AUD . | Use of other subs . | Structures compared . | Findings . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| De Bellis et al. (2000) | MRI | Semi- automated ROI | 5:7/10:14 | 17.2 (2.2)/17.0 (2.4) Range: 13.5–21.0 | Substance misuse diagnoses made using modified version of DSM-IV Seven lifetime alcohol dependence, five lifetime alcohol abuse. Majority comorbid Axis I disorders, most commonly depression, conduct disorder or PTSD Excluded if medically significant medical or neurological illness or learning disability | No use for 2 weeks prior to scan | No data | No data | Majority (9) history of cannabis abuse or dependence. Smaller numbers had used other substances. Tobacco use not detailed | Total grey and white matter volumes Amygdala, hippocampal and lateral ventricular volumes. Corpus callosum area | Reduced L, R and combined hippocampal volumes (F = 7.06, P = 0.01, d = 0.97; F = 5.04, P = 0.03, d = 0.82; and F = 6.47, P < 0.02, d = 0.93, respectively) Difference not significant when five subjects with PTSD excluded from comparison (F = 4.18, P < 0.06, d = 0.91). No difference total grey and white matter volumes, amygdalar volumes or corpus callosal area |
| De Bellis et al. (2005) | MRI | Semi- automated ROI | 8:6/16:12 | 17.0 (2.1)/16.9 (2.3) Range: 13.5–21.0 | DSM-IV alcohol abuse or dependence. Majority had Axis I comorbidity, most commonly depression or conduct disorder Excluded if medically significant medical or neurological illness or learning disability | No alcohol use in 12 h preceding scan | Mean number of drinks per maximum drinking episode 12.1 ± 0.86 | Age onset AUD: 15.6 (2.4) | Majority of AUD group (11) also had history of cannabis use disorder. Small numbers had used other substances, but not within 2 weeks of scan. Tobacco use not detailed | Total cerebral volume, total PFC volume, grey and white matter PFC volume, volume of thalamus, brainstem, right and left cerebellum | Reduced total PFC (t = −2.38, P = 0.02, d = 0.80) and PFC white matter volumes (t = −2.38, P < .007, d = 0.96) Findings persisted on controlling for cerebral volume but not comorbidity. When both controlled for d = 0.55 and 0.52 for total PFC and PFC white matter, respectively |
| Nagel et al. (2005) | MRI | Semi- automated ROI | 9:5/5:7 | 16.8 (0.7)/16.5 (0.9) Range: 15.2–17.9 | Met DSM-IV criteria for alcohol abuse or dependence. Only additional DSM-IV diagnosis mild-to-moderate conduct disorder (present in 5) Excluded if learning disability, serious medical or neurological problems, significant head injury or psychotropic medication | Abstinent from the use of alcohol or other drugs for 5 days | Mean drinks/month in last 3 months: 43.00 (31.89) | Regular drinking began 14.9 (1.1) years | AUD group did not differ from controls in the family history of substance use disorder, lifetime use of other drugs, recent marijuana use or marijuana abuse/dependence. Significant greater lifetime episodes of marijuana use and cigarettes in the last month | Whole brain grey and white matter volumes Hippocampal volumes | Reduced left hippocampal volume (t = 2.90, P < 0.01, d = 1.08), no reduction in right hippocampal volume (t = 0.51, P = 0.61, d = 0.19) Remained significant on excluding comorbid conduct disorder (t = 1.71, P < 0.05, d = 0.70) |
| De Bellis et al. (2008) | MRI DTI | Voxel-based ROI | 25:7/17:11 | 16.9 (1.2)/15.9 (1.1)b Range: 13.3–19.3 | Recruited from treatment centres. Met DSM-IV criteria for lifetime alcohol dependence or current alcohol abuse. Majority had Axis I comorbidity, most commonly oppositional defiant disorder, depression, attention deficit hyperactivity disorder or conduct disorder Excluded if medically significant medical or neurological illness or learning disability | Mean (SD) number of days since last consumed alcohol 63.7 (88.2) | Mean number of drinks per maximum drinking episode 13.2 ± 8.6 | Regular drinking began 15.6 (1.3) years AUD onset: 14.7 (1.3) years | Majority (24) of AUD group also had history of cannabis use disorder and had smoked cigarettes. Small numbers had used other substances | Corpus callosum divided into seven regions: rostrum, genu, rostral body, anterior mid-body, posterior mid-body, isthmus and splenium. Microstructural integrity of each region compared | FA values in the corpus callosum rostral body higher in the AUD group than in the control group, though not significant after adjusting for age and sex (F = 2.9, P = 0.09, d = 0.4) FA values also higher in the isthmus in the AUD group, which was significant after controlling for age and sex (d = 0.9) |
| Medina et al. (2008) | MRI | Semi- automated ROI | 9:5/5:7 | M: 16.6 (0.7)/16.6 (0.7) F: 17.1 (0.6)/16.5 (1.0) Range: 15.2–17.9 | Met DSM-IV criteria for alcohol abuse or dependence. Only additional DSM-IV diagnosis mild-to-moderate conduct disorder (present in 5) Excluded if learning disability, serious medical or neurological problems, significant head injury or psychotropic medication | Abstinent from the use of alcohol or other drugs for 5 days | Mean drinks/month in last 3 months: 43.00 (31.89) | Regular drinking began 14.9 (1.1) years | AUD group did not differ from controls in the family history of substance use disorder, lifetime use of other drugs, recent marijuana use or marijuana abuse/dependence criteria. Significantly, greater lifetime episodes of marijuana use and use of cigarettes in the last month | PFC volume. Total and divided into following regions: posterior, anterior dorsal and anterior ventral. Also, white matter volume divided into same subregions | Whole group comparison: no significant difference in PFC volume (whole or white matter) between groups. On comparing gender sub-groups, however, females with AUD demonstrated smaller PFC volumes, while males with AUD had larger PFC volumes. The same pattern was observed for PFC white matter volumes Significant group-by-gender interaction for total PFC volume (F = 10.63, P < 0.003, 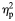 = 0.29), and PFC white matter volume (F = 5.64, P < 0.03, = 0.29), and PFC white matter volume (F = 5.64, P < 0.03, 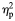 = 0.17). = 0.17). |
Intracranial volume was adjusted for in all volumetric analyses except where otherwise specified.
Effect sizes reported as Cohen's d aside unless otherwise stated. 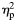 = partial eta squared.
= partial eta squared.
AUD, alcohol use disorder; encompasses either alcohol abuse or dependence; AD, alcohol dependence.
aIf genders have been analysed separately, mean age is displayed separately for each gender. Otherwise mean age is combined.
bSignificant difference between groups.
The Duke group compared treatment samples of mixed-gender groups of adolescents with AUD (defined as DSM-IV alcohol abuse or dependence) and other Axis I disorders [including conduct, depressive, attention deficit hyperactivity, post-traumatic stress disorder (PTSD), generalized anxiety, bipolar and cannabis and hallucinogen use disorders] with controls without a history of AUD. Cases and controls were not matched for the family history of substance use, and Axis II disorders are not discussed. Each report examined different brain regions, the first two using region of interest (ROI)-based volumetric methodology, the most recent augmenting this with DTI. The first study reported that both left and right hippocampi were significantly smaller in subjects with AUDs, the effect being slightly stronger on the left (Cohen's d of 0.93 vs. 0.82). Intracranial and cerebral volumes, cortical grey and white matter, amygdala volumes, lateral ventricular volumes and corpus callosum area did not differ between groups (De Bellis et al., 2000). The second paper reported smaller PFC total and white matter volumes and increased PFC cerebral spinal fluid in adolescents with AUD, with no gender-by-group interaction (De Bellis et al., 2005). The first two findings were not significant after controlling for comorbid disorders (P = 0.1 for both and Cohen's d = 0.55 and 0.52, respectively), though PFC cerebral spinal fluid volumes were still significantly increased in the AUD group. Comorbid disorders were not themselves associated with reduced PFC volumes, leading the authors to suggest that the loss of significant difference in PFC volume/white matter volume on controlling for comorbidity was due to loss of power. No difference was observed in thalamic, pons/brainstem and cerebellar volumes. PFC volume variables significantly and negatively correlated with the most number of drinks per maximum drinking episode. The most recent study by De Bellis et al. (2008) used DTI to compare white matter integrity in corpus callosal regions in a larger group of adolescents (32) with AUD and controls. Somewhat surprisingly, FA was higher in the rostral body and isthmal regions in the AUD group compared with controls; the former finding remained significant after controlling for age and sex, though the latter did not. Controlling for age was essential as the AUD group was significantly older. The authors suggest that these unexpected findings might be explained by adolescents with AUD having a pre-morbid vulnerability for accelerated PFC and temporo-parietal maturation, thus increasing their risk for adolescent substance use disorders.
Results for young adult systematic review
The seven studies fulfilling inclusion criteria were cross-sectional and are summarized in Table 2. Several themes were evident. First, even though studies met the mean age inclusion criterion, many individuals were substantially >40. In the Agartz et al. (1999) study, for example, the oldest subject was 59. Given the established age–alcohol interaction in most of the brain regions (Pfefferbaum et al., 1992), these older individuals could conceivably be driving the differences seen.
Studies investigating structural imaging abnormalities in adult problem drinkers aged under 40
| Study . | Imaging modality . | Image analysis . | GenderAUD/Cont. (M:F) . | Mean age (SD) AUD/Cont. (M/F)a . | Inclusion criteria . | Drinking status . | Estimate of alcohol quantities consumed (kg) . | Mean age of onset (SD) of alcohol use/AUD . | Use of other subs . | Structures compared . | Findings . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hommer et al. (1996) | MRI | Semi- automated ROI | 13:14/10:9 | M: 39.3 (7.2)/37.1 (5.6) F: 38.9 (6.7)/35.9 (4.7) Range: 30–50 | Inpatient or outpatient treatment for DSM-III-R AD. No evidence psychotic, cognitive, neurological disorder, ‘overt liver disease’ or nutritional deficiency. No history of head injury requiring hospitalization | Abstained at least 10 days | Mean estimated lifetime alcohol consumption. Men: 463 (453) kg Women: 403 (560) kg | Mean age onset of daily heavy alcohol consumption. Men: 26.0 Women: 30.7 | Excluded any other substance abuse disorder in past 6 months, but seven lifetime history of other substance dependence and majority smokers | Cross-sectional area of the corpus callosum | Corpus callosum area was significantly smaller in alcoholic women than control women sex (F = 13.1, P < 0.001, d = 1.62). No significant difference was observed in alcoholic men (F = 0.6, P = 0.42, d = 0.34) |
| Pfefferbaum et al. (1997) | MRI | Semi- automated ROI | 33:0/65:0 | 37.5 (4.5)/32.9 (6.5) Range: 26–44 | Hospitalized for alcoholism (meeting research diagnostic criteria for same). No history of major affective disorder, schizophrenia, medical conditions affecting CNS, seizure disorder unrelated to alcohol withdrawal, substance abuse other than alcohol in the last year | Four weeks after admission | Mean estimated lifetime alcohol consumption 1320.3 (820.1) | Mean age of ‘disease onset’: 20.3 | Excluded if any other substance abuse disorder in the past year. No data on study or control group tobacco use | Volumes of: total grey and white matter; ventricles; prefrontal; frontal; anterior superior temporal; posterior superior temporal; occipital | Lateral and third ventricular enlargement Borderline significant grey matter deficit in the fontal cortex (F = 4.1, P < 0.05, d = 0.44) No differences in white matter volumes (F = .08, P = 0.77, d = 0.06) The age imbalance was controlled for in the analyses |
| Agartz et al. (1999) | MRI | Semi- automated ROI | 26:26/17:19 | M: 36.9 (6.2)/35.7 (8.2) F: 37.4 (5.6)/35.6 (7.9) Range: 27–53 | Hospitalized for DSM-III-R alcohol dependence Subjects: no history psychosis, ‘somatic disease’, DTs, IQ < 80, dementia or Korsakoff's. Controls: no psychiatric disorder meeting DSM-III-R criteria | Abstained for at least 3 weeks | Lifetime drinking, kg Men: 624.7 (555.2) Women: 360.3 (476.9) | Age of onset of heavy drinking. Men: 23.3 (6.0) Women: 26.2 (12.2) | Excluded if other substance abuse in preceding 6 months (except tobacco) | Hippocampal volume Non-hippocampal brain volume | R hippocampus volume reduced in AD men (F = 7.8, P = < 0.008, d = 0.89) and women (F = 9.52, P = 0.004, d = 0.95)c L hippocampus volume reduced in AD women (F = 7.2, P = 0.01, d = 0.83), but not men (F = 1.5, P = 0.23, d = 0.39)c Non-hippocampal brain volume reduced in women (F = 14.1, P < 0.001, d = 1.16), but not men (F = 2.2, P = 0.15, d = 0.47)c The reductions in hippocampal volume were proportional to the reduction in volume of the rest of the brain (i.e. differences non-significant on controlling for whole brain volume) |
| Fein et al. (2002) | MRI | Voxel-based ROI | 24:0/17:0 The age-balanced sample was 16:0/17:0 | 38.7(9.0)/30.0(5.2) Range: 25–54 AD (those >42 excluded for age matched comparison) | Recruited from community, treatment naïve, but meeting DSM-IV-R criteria for alcohol dependence. Excluded if history of substance abuse other than alcohol, major psychiatric or neurological disorder (head injury with loss of consciousness), medical conditions that may affect brain structure, (including diabetes, HIV, and lung, kidney, heart disease) | Not stated. Likely drank shortly before scan | Lifetime total number of drinks: 58,583 (48,041) | Age onset of drinking: 15.0 | Excluded if history of substance abuse other than alcohol | Fifteen cortical regions of interest, encompassing the whole of cortex | Significam=ntly reduced total cortical, posterior prefrontal, dorsolateral prefrontal, lateral parietal and mesial parietal grey matter volume Only findings in posterior and dorsolateral prefrontal regions remained significantly in analysis of age-balanced subset No difference in total and regional white matter volumes (either in comparison of all subjects or just age-balanced subset) |
| Agartz et al. (2003) | MRI | Semi- automated ROI | 40:14/17:3 | 38.3/39.3 Range: 27–59 | Hospitalized for DSM-III-R AD Subjects: no history psychosis, ‘somatic disease’, DTs, IQ < 80, dementia or Korsakoff's. Controls: no psychiatric disorder meeting DSM-III-R criteria | Abstained for at least 3 weeks | Lifetime drinking (kg) Men: 531.5 (499.4) Women: 432.1 (493.1) | Age of onset alcohol dependence. Men: 26.5 (8.5) Women: 31.2 (10.5) | Excluded if used in preceding 6 months (except tobacco) | Volumes of total grey and white matter, hippocampi and lateral ventricules. Corpus callosum area (measured from mid- saggital slice) | Reduced total grey and white matter and corpus callosum area (F = 7.7, P = 0.007, d = 0.74; F = 9.0, P = 0.004, d = 0.80; F = 9.3, P = 0.003, d = 0.81, respectively) and increased CSF volumes (F = 9.5, P = 0.003, d = 0.82). White matter effect particularly pronounced in women; gender effect for white matter volume, (F = 18.65, P < 0.001, d = 1.15) No significant difference in ventricular or hippocampal volumes |
| Fein et al. (2010) | MRI | Automated ROI | 49:35/39:28 | M: 31.4 (7.8) 32.8 (8.3)/ F: 30.7 (7.8)/32.4 (8.8) Range: 19–51 | Recruited from community, treatment naïve, but meeting DSM-IV-R criteria for alcohol dependence. Excluded if history of other substance abuse, major psychiatric or neurological disorder (including head trauma or Wernicke–Korsakoff syndrome), history of diabetes, stroke or hypertension that required medical intervention | Breath-alyzer confirmed abstinence before scanning. Likely drinking shortly before this | Lifetime alcohol use (standard drinks): Men: 17,600 (11,500) Women: 11,600 (7300) | Age of onset of drinking Men: 16.2 Women: 16.0 | Excluded if history of substance abuse other than alcohol (except tobacco) | Total grey matter volume and volume of the five major lobes (frontal, limbic, occipital, parietal and temporal) | No significant group differences on controlling for sex, age and cranial volume |
| Rando et al. (2011) | MRI | Voxel- based ROI | 35:10/28:22 | 38.2 (7.7)/31.1 (9.0)b Range: 18–50 | Hospitalized for DSM-IV alcohol dependence. Excluded if taking any medication for medical or psychiatric problems | Five weeks after admission | Average 19.4 drinks per day for 18.6 years | Age of onset of drinking: 19.6 | Excluded if current diagnosis of dependence (except tobacco) | Whole brain grey matter | Reduced grey matter volume in: lateral PFC; (including the right dorsolateral and inferolateral PFC); medial frontal cortex (including the dorsal anterior cingulate gyrus and medial and lateral superior frontal gyrus); the middle frontal gyrus; a posterior region centred on the parietal–occipital sulcus, overlapping the precuneus, cuneus, and posterior cingulate regions |
| Study . | Imaging modality . | Image analysis . | GenderAUD/Cont. (M:F) . | Mean age (SD) AUD/Cont. (M/F)a . | Inclusion criteria . | Drinking status . | Estimate of alcohol quantities consumed (kg) . | Mean age of onset (SD) of alcohol use/AUD . | Use of other subs . | Structures compared . | Findings . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hommer et al. (1996) | MRI | Semi- automated ROI | 13:14/10:9 | M: 39.3 (7.2)/37.1 (5.6) F: 38.9 (6.7)/35.9 (4.7) Range: 30–50 | Inpatient or outpatient treatment for DSM-III-R AD. No evidence psychotic, cognitive, neurological disorder, ‘overt liver disease’ or nutritional deficiency. No history of head injury requiring hospitalization | Abstained at least 10 days | Mean estimated lifetime alcohol consumption. Men: 463 (453) kg Women: 403 (560) kg | Mean age onset of daily heavy alcohol consumption. Men: 26.0 Women: 30.7 | Excluded any other substance abuse disorder in past 6 months, but seven lifetime history of other substance dependence and majority smokers | Cross-sectional area of the corpus callosum | Corpus callosum area was significantly smaller in alcoholic women than control women sex (F = 13.1, P < 0.001, d = 1.62). No significant difference was observed in alcoholic men (F = 0.6, P = 0.42, d = 0.34) |
| Pfefferbaum et al. (1997) | MRI | Semi- automated ROI | 33:0/65:0 | 37.5 (4.5)/32.9 (6.5) Range: 26–44 | Hospitalized for alcoholism (meeting research diagnostic criteria for same). No history of major affective disorder, schizophrenia, medical conditions affecting CNS, seizure disorder unrelated to alcohol withdrawal, substance abuse other than alcohol in the last year | Four weeks after admission | Mean estimated lifetime alcohol consumption 1320.3 (820.1) | Mean age of ‘disease onset’: 20.3 | Excluded if any other substance abuse disorder in the past year. No data on study or control group tobacco use | Volumes of: total grey and white matter; ventricles; prefrontal; frontal; anterior superior temporal; posterior superior temporal; occipital | Lateral and third ventricular enlargement Borderline significant grey matter deficit in the fontal cortex (F = 4.1, P < 0.05, d = 0.44) No differences in white matter volumes (F = .08, P = 0.77, d = 0.06) The age imbalance was controlled for in the analyses |
| Agartz et al. (1999) | MRI | Semi- automated ROI | 26:26/17:19 | M: 36.9 (6.2)/35.7 (8.2) F: 37.4 (5.6)/35.6 (7.9) Range: 27–53 | Hospitalized for DSM-III-R alcohol dependence Subjects: no history psychosis, ‘somatic disease’, DTs, IQ < 80, dementia or Korsakoff's. Controls: no psychiatric disorder meeting DSM-III-R criteria | Abstained for at least 3 weeks | Lifetime drinking, kg Men: 624.7 (555.2) Women: 360.3 (476.9) | Age of onset of heavy drinking. Men: 23.3 (6.0) Women: 26.2 (12.2) | Excluded if other substance abuse in preceding 6 months (except tobacco) | Hippocampal volume Non-hippocampal brain volume | R hippocampus volume reduced in AD men (F = 7.8, P = < 0.008, d = 0.89) and women (F = 9.52, P = 0.004, d = 0.95)c L hippocampus volume reduced in AD women (F = 7.2, P = 0.01, d = 0.83), but not men (F = 1.5, P = 0.23, d = 0.39)c Non-hippocampal brain volume reduced in women (F = 14.1, P < 0.001, d = 1.16), but not men (F = 2.2, P = 0.15, d = 0.47)c The reductions in hippocampal volume were proportional to the reduction in volume of the rest of the brain (i.e. differences non-significant on controlling for whole brain volume) |
| Fein et al. (2002) | MRI | Voxel-based ROI | 24:0/17:0 The age-balanced sample was 16:0/17:0 | 38.7(9.0)/30.0(5.2) Range: 25–54 AD (those >42 excluded for age matched comparison) | Recruited from community, treatment naïve, but meeting DSM-IV-R criteria for alcohol dependence. Excluded if history of substance abuse other than alcohol, major psychiatric or neurological disorder (head injury with loss of consciousness), medical conditions that may affect brain structure, (including diabetes, HIV, and lung, kidney, heart disease) | Not stated. Likely drank shortly before scan | Lifetime total number of drinks: 58,583 (48,041) | Age onset of drinking: 15.0 | Excluded if history of substance abuse other than alcohol | Fifteen cortical regions of interest, encompassing the whole of cortex | Significam=ntly reduced total cortical, posterior prefrontal, dorsolateral prefrontal, lateral parietal and mesial parietal grey matter volume Only findings in posterior and dorsolateral prefrontal regions remained significantly in analysis of age-balanced subset No difference in total and regional white matter volumes (either in comparison of all subjects or just age-balanced subset) |
| Agartz et al. (2003) | MRI | Semi- automated ROI | 40:14/17:3 | 38.3/39.3 Range: 27–59 | Hospitalized for DSM-III-R AD Subjects: no history psychosis, ‘somatic disease’, DTs, IQ < 80, dementia or Korsakoff's. Controls: no psychiatric disorder meeting DSM-III-R criteria | Abstained for at least 3 weeks | Lifetime drinking (kg) Men: 531.5 (499.4) Women: 432.1 (493.1) | Age of onset alcohol dependence. Men: 26.5 (8.5) Women: 31.2 (10.5) | Excluded if used in preceding 6 months (except tobacco) | Volumes of total grey and white matter, hippocampi and lateral ventricules. Corpus callosum area (measured from mid- saggital slice) | Reduced total grey and white matter and corpus callosum area (F = 7.7, P = 0.007, d = 0.74; F = 9.0, P = 0.004, d = 0.80; F = 9.3, P = 0.003, d = 0.81, respectively) and increased CSF volumes (F = 9.5, P = 0.003, d = 0.82). White matter effect particularly pronounced in women; gender effect for white matter volume, (F = 18.65, P < 0.001, d = 1.15) No significant difference in ventricular or hippocampal volumes |
| Fein et al. (2010) | MRI | Automated ROI | 49:35/39:28 | M: 31.4 (7.8) 32.8 (8.3)/ F: 30.7 (7.8)/32.4 (8.8) Range: 19–51 | Recruited from community, treatment naïve, but meeting DSM-IV-R criteria for alcohol dependence. Excluded if history of other substance abuse, major psychiatric or neurological disorder (including head trauma or Wernicke–Korsakoff syndrome), history of diabetes, stroke or hypertension that required medical intervention | Breath-alyzer confirmed abstinence before scanning. Likely drinking shortly before this | Lifetime alcohol use (standard drinks): Men: 17,600 (11,500) Women: 11,600 (7300) | Age of onset of drinking Men: 16.2 Women: 16.0 | Excluded if history of substance abuse other than alcohol (except tobacco) | Total grey matter volume and volume of the five major lobes (frontal, limbic, occipital, parietal and temporal) | No significant group differences on controlling for sex, age and cranial volume |
| Rando et al. (2011) | MRI | Voxel- based ROI | 35:10/28:22 | 38.2 (7.7)/31.1 (9.0)b Range: 18–50 | Hospitalized for DSM-IV alcohol dependence. Excluded if taking any medication for medical or psychiatric problems | Five weeks after admission | Average 19.4 drinks per day for 18.6 years | Age of onset of drinking: 19.6 | Excluded if current diagnosis of dependence (except tobacco) | Whole brain grey matter | Reduced grey matter volume in: lateral PFC; (including the right dorsolateral and inferolateral PFC); medial frontal cortex (including the dorsal anterior cingulate gyrus and medial and lateral superior frontal gyrus); the middle frontal gyrus; a posterior region centred on the parietal–occipital sulcus, overlapping the precuneus, cuneus, and posterior cingulate regions |
When calculable, effect sizes reported as Cohen's d.
AD, alcohol dependence; DTs, delirium tremens.
aIf genders have been analysed separately, mean age is displayed separately for each gender. Otherwise mean age is combined.
bSignificant difference between groups.
cUnless specifically stated intracranial volume was controlled for in analyses.
Studies investigating structural imaging abnormalities in adult problem drinkers aged under 40
| Study . | Imaging modality . | Image analysis . | GenderAUD/Cont. (M:F) . | Mean age (SD) AUD/Cont. (M/F)a . | Inclusion criteria . | Drinking status . | Estimate of alcohol quantities consumed (kg) . | Mean age of onset (SD) of alcohol use/AUD . | Use of other subs . | Structures compared . | Findings . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hommer et al. (1996) | MRI | Semi- automated ROI | 13:14/10:9 | M: 39.3 (7.2)/37.1 (5.6) F: 38.9 (6.7)/35.9 (4.7) Range: 30–50 | Inpatient or outpatient treatment for DSM-III-R AD. No evidence psychotic, cognitive, neurological disorder, ‘overt liver disease’ or nutritional deficiency. No history of head injury requiring hospitalization | Abstained at least 10 days | Mean estimated lifetime alcohol consumption. Men: 463 (453) kg Women: 403 (560) kg | Mean age onset of daily heavy alcohol consumption. Men: 26.0 Women: 30.7 | Excluded any other substance abuse disorder in past 6 months, but seven lifetime history of other substance dependence and majority smokers | Cross-sectional area of the corpus callosum | Corpus callosum area was significantly smaller in alcoholic women than control women sex (F = 13.1, P < 0.001, d = 1.62). No significant difference was observed in alcoholic men (F = 0.6, P = 0.42, d = 0.34) |
| Pfefferbaum et al. (1997) | MRI | Semi- automated ROI | 33:0/65:0 | 37.5 (4.5)/32.9 (6.5) Range: 26–44 | Hospitalized for alcoholism (meeting research diagnostic criteria for same). No history of major affective disorder, schizophrenia, medical conditions affecting CNS, seizure disorder unrelated to alcohol withdrawal, substance abuse other than alcohol in the last year | Four weeks after admission | Mean estimated lifetime alcohol consumption 1320.3 (820.1) | Mean age of ‘disease onset’: 20.3 | Excluded if any other substance abuse disorder in the past year. No data on study or control group tobacco use | Volumes of: total grey and white matter; ventricles; prefrontal; frontal; anterior superior temporal; posterior superior temporal; occipital | Lateral and third ventricular enlargement Borderline significant grey matter deficit in the fontal cortex (F = 4.1, P < 0.05, d = 0.44) No differences in white matter volumes (F = .08, P = 0.77, d = 0.06) The age imbalance was controlled for in the analyses |
| Agartz et al. (1999) | MRI | Semi- automated ROI | 26:26/17:19 | M: 36.9 (6.2)/35.7 (8.2) F: 37.4 (5.6)/35.6 (7.9) Range: 27–53 | Hospitalized for DSM-III-R alcohol dependence Subjects: no history psychosis, ‘somatic disease’, DTs, IQ < 80, dementia or Korsakoff's. Controls: no psychiatric disorder meeting DSM-III-R criteria | Abstained for at least 3 weeks | Lifetime drinking, kg Men: 624.7 (555.2) Women: 360.3 (476.9) | Age of onset of heavy drinking. Men: 23.3 (6.0) Women: 26.2 (12.2) | Excluded if other substance abuse in preceding 6 months (except tobacco) | Hippocampal volume Non-hippocampal brain volume | R hippocampus volume reduced in AD men (F = 7.8, P = < 0.008, d = 0.89) and women (F = 9.52, P = 0.004, d = 0.95)c L hippocampus volume reduced in AD women (F = 7.2, P = 0.01, d = 0.83), but not men (F = 1.5, P = 0.23, d = 0.39)c Non-hippocampal brain volume reduced in women (F = 14.1, P < 0.001, d = 1.16), but not men (F = 2.2, P = 0.15, d = 0.47)c The reductions in hippocampal volume were proportional to the reduction in volume of the rest of the brain (i.e. differences non-significant on controlling for whole brain volume) |
| Fein et al. (2002) | MRI | Voxel-based ROI | 24:0/17:0 The age-balanced sample was 16:0/17:0 | 38.7(9.0)/30.0(5.2) Range: 25–54 AD (those >42 excluded for age matched comparison) | Recruited from community, treatment naïve, but meeting DSM-IV-R criteria for alcohol dependence. Excluded if history of substance abuse other than alcohol, major psychiatric or neurological disorder (head injury with loss of consciousness), medical conditions that may affect brain structure, (including diabetes, HIV, and lung, kidney, heart disease) | Not stated. Likely drank shortly before scan | Lifetime total number of drinks: 58,583 (48,041) | Age onset of drinking: 15.0 | Excluded if history of substance abuse other than alcohol | Fifteen cortical regions of interest, encompassing the whole of cortex | Significam=ntly reduced total cortical, posterior prefrontal, dorsolateral prefrontal, lateral parietal and mesial parietal grey matter volume Only findings in posterior and dorsolateral prefrontal regions remained significantly in analysis of age-balanced subset No difference in total and regional white matter volumes (either in comparison of all subjects or just age-balanced subset) |
| Agartz et al. (2003) | MRI | Semi- automated ROI | 40:14/17:3 | 38.3/39.3 Range: 27–59 | Hospitalized for DSM-III-R AD Subjects: no history psychosis, ‘somatic disease’, DTs, IQ < 80, dementia or Korsakoff's. Controls: no psychiatric disorder meeting DSM-III-R criteria | Abstained for at least 3 weeks | Lifetime drinking (kg) Men: 531.5 (499.4) Women: 432.1 (493.1) | Age of onset alcohol dependence. Men: 26.5 (8.5) Women: 31.2 (10.5) | Excluded if used in preceding 6 months (except tobacco) | Volumes of total grey and white matter, hippocampi and lateral ventricules. Corpus callosum area (measured from mid- saggital slice) | Reduced total grey and white matter and corpus callosum area (F = 7.7, P = 0.007, d = 0.74; F = 9.0, P = 0.004, d = 0.80; F = 9.3, P = 0.003, d = 0.81, respectively) and increased CSF volumes (F = 9.5, P = 0.003, d = 0.82). White matter effect particularly pronounced in women; gender effect for white matter volume, (F = 18.65, P < 0.001, d = 1.15) No significant difference in ventricular or hippocampal volumes |
| Fein et al. (2010) | MRI | Automated ROI | 49:35/39:28 | M: 31.4 (7.8) 32.8 (8.3)/ F: 30.7 (7.8)/32.4 (8.8) Range: 19–51 | Recruited from community, treatment naïve, but meeting DSM-IV-R criteria for alcohol dependence. Excluded if history of other substance abuse, major psychiatric or neurological disorder (including head trauma or Wernicke–Korsakoff syndrome), history of diabetes, stroke or hypertension that required medical intervention | Breath-alyzer confirmed abstinence before scanning. Likely drinking shortly before this | Lifetime alcohol use (standard drinks): Men: 17,600 (11,500) Women: 11,600 (7300) | Age of onset of drinking Men: 16.2 Women: 16.0 | Excluded if history of substance abuse other than alcohol (except tobacco) | Total grey matter volume and volume of the five major lobes (frontal, limbic, occipital, parietal and temporal) | No significant group differences on controlling for sex, age and cranial volume |
| Rando et al. (2011) | MRI | Voxel- based ROI | 35:10/28:22 | 38.2 (7.7)/31.1 (9.0)b Range: 18–50 | Hospitalized for DSM-IV alcohol dependence. Excluded if taking any medication for medical or psychiatric problems | Five weeks after admission | Average 19.4 drinks per day for 18.6 years | Age of onset of drinking: 19.6 | Excluded if current diagnosis of dependence (except tobacco) | Whole brain grey matter | Reduced grey matter volume in: lateral PFC; (including the right dorsolateral and inferolateral PFC); medial frontal cortex (including the dorsal anterior cingulate gyrus and medial and lateral superior frontal gyrus); the middle frontal gyrus; a posterior region centred on the parietal–occipital sulcus, overlapping the precuneus, cuneus, and posterior cingulate regions |
| Study . | Imaging modality . | Image analysis . | GenderAUD/Cont. (M:F) . | Mean age (SD) AUD/Cont. (M/F)a . | Inclusion criteria . | Drinking status . | Estimate of alcohol quantities consumed (kg) . | Mean age of onset (SD) of alcohol use/AUD . | Use of other subs . | Structures compared . | Findings . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hommer et al. (1996) | MRI | Semi- automated ROI | 13:14/10:9 | M: 39.3 (7.2)/37.1 (5.6) F: 38.9 (6.7)/35.9 (4.7) Range: 30–50 | Inpatient or outpatient treatment for DSM-III-R AD. No evidence psychotic, cognitive, neurological disorder, ‘overt liver disease’ or nutritional deficiency. No history of head injury requiring hospitalization | Abstained at least 10 days | Mean estimated lifetime alcohol consumption. Men: 463 (453) kg Women: 403 (560) kg | Mean age onset of daily heavy alcohol consumption. Men: 26.0 Women: 30.7 | Excluded any other substance abuse disorder in past 6 months, but seven lifetime history of other substance dependence and majority smokers | Cross-sectional area of the corpus callosum | Corpus callosum area was significantly smaller in alcoholic women than control women sex (F = 13.1, P < 0.001, d = 1.62). No significant difference was observed in alcoholic men (F = 0.6, P = 0.42, d = 0.34) |
| Pfefferbaum et al. (1997) | MRI | Semi- automated ROI | 33:0/65:0 | 37.5 (4.5)/32.9 (6.5) Range: 26–44 | Hospitalized for alcoholism (meeting research diagnostic criteria for same). No history of major affective disorder, schizophrenia, medical conditions affecting CNS, seizure disorder unrelated to alcohol withdrawal, substance abuse other than alcohol in the last year | Four weeks after admission | Mean estimated lifetime alcohol consumption 1320.3 (820.1) | Mean age of ‘disease onset’: 20.3 | Excluded if any other substance abuse disorder in the past year. No data on study or control group tobacco use | Volumes of: total grey and white matter; ventricles; prefrontal; frontal; anterior superior temporal; posterior superior temporal; occipital | Lateral and third ventricular enlargement Borderline significant grey matter deficit in the fontal cortex (F = 4.1, P < 0.05, d = 0.44) No differences in white matter volumes (F = .08, P = 0.77, d = 0.06) The age imbalance was controlled for in the analyses |
| Agartz et al. (1999) | MRI | Semi- automated ROI | 26:26/17:19 | M: 36.9 (6.2)/35.7 (8.2) F: 37.4 (5.6)/35.6 (7.9) Range: 27–53 | Hospitalized for DSM-III-R alcohol dependence Subjects: no history psychosis, ‘somatic disease’, DTs, IQ < 80, dementia or Korsakoff's. Controls: no psychiatric disorder meeting DSM-III-R criteria | Abstained for at least 3 weeks | Lifetime drinking, kg Men: 624.7 (555.2) Women: 360.3 (476.9) | Age of onset of heavy drinking. Men: 23.3 (6.0) Women: 26.2 (12.2) | Excluded if other substance abuse in preceding 6 months (except tobacco) | Hippocampal volume Non-hippocampal brain volume | R hippocampus volume reduced in AD men (F = 7.8, P = < 0.008, d = 0.89) and women (F = 9.52, P = 0.004, d = 0.95)c L hippocampus volume reduced in AD women (F = 7.2, P = 0.01, d = 0.83), but not men (F = 1.5, P = 0.23, d = 0.39)c Non-hippocampal brain volume reduced in women (F = 14.1, P < 0.001, d = 1.16), but not men (F = 2.2, P = 0.15, d = 0.47)c The reductions in hippocampal volume were proportional to the reduction in volume of the rest of the brain (i.e. differences non-significant on controlling for whole brain volume) |
| Fein et al. (2002) | MRI | Voxel-based ROI | 24:0/17:0 The age-balanced sample was 16:0/17:0 | 38.7(9.0)/30.0(5.2) Range: 25–54 AD (those >42 excluded for age matched comparison) | Recruited from community, treatment naïve, but meeting DSM-IV-R criteria for alcohol dependence. Excluded if history of substance abuse other than alcohol, major psychiatric or neurological disorder (head injury with loss of consciousness), medical conditions that may affect brain structure, (including diabetes, HIV, and lung, kidney, heart disease) | Not stated. Likely drank shortly before scan | Lifetime total number of drinks: 58,583 (48,041) | Age onset of drinking: 15.0 | Excluded if history of substance abuse other than alcohol | Fifteen cortical regions of interest, encompassing the whole of cortex | Significam=ntly reduced total cortical, posterior prefrontal, dorsolateral prefrontal, lateral parietal and mesial parietal grey matter volume Only findings in posterior and dorsolateral prefrontal regions remained significantly in analysis of age-balanced subset No difference in total and regional white matter volumes (either in comparison of all subjects or just age-balanced subset) |
| Agartz et al. (2003) | MRI | Semi- automated ROI | 40:14/17:3 | 38.3/39.3 Range: 27–59 | Hospitalized for DSM-III-R AD Subjects: no history psychosis, ‘somatic disease’, DTs, IQ < 80, dementia or Korsakoff's. Controls: no psychiatric disorder meeting DSM-III-R criteria | Abstained for at least 3 weeks | Lifetime drinking (kg) Men: 531.5 (499.4) Women: 432.1 (493.1) | Age of onset alcohol dependence. Men: 26.5 (8.5) Women: 31.2 (10.5) | Excluded if used in preceding 6 months (except tobacco) | Volumes of total grey and white matter, hippocampi and lateral ventricules. Corpus callosum area (measured from mid- saggital slice) | Reduced total grey and white matter and corpus callosum area (F = 7.7, P = 0.007, d = 0.74; F = 9.0, P = 0.004, d = 0.80; F = 9.3, P = 0.003, d = 0.81, respectively) and increased CSF volumes (F = 9.5, P = 0.003, d = 0.82). White matter effect particularly pronounced in women; gender effect for white matter volume, (F = 18.65, P < 0.001, d = 1.15) No significant difference in ventricular or hippocampal volumes |
| Fein et al. (2010) | MRI | Automated ROI | 49:35/39:28 | M: 31.4 (7.8) 32.8 (8.3)/ F: 30.7 (7.8)/32.4 (8.8) Range: 19–51 | Recruited from community, treatment naïve, but meeting DSM-IV-R criteria for alcohol dependence. Excluded if history of other substance abuse, major psychiatric or neurological disorder (including head trauma or Wernicke–Korsakoff syndrome), history of diabetes, stroke or hypertension that required medical intervention | Breath-alyzer confirmed abstinence before scanning. Likely drinking shortly before this | Lifetime alcohol use (standard drinks): Men: 17,600 (11,500) Women: 11,600 (7300) | Age of onset of drinking Men: 16.2 Women: 16.0 | Excluded if history of substance abuse other than alcohol (except tobacco) | Total grey matter volume and volume of the five major lobes (frontal, limbic, occipital, parietal and temporal) | No significant group differences on controlling for sex, age and cranial volume |
| Rando et al. (2011) | MRI | Voxel- based ROI | 35:10/28:22 | 38.2 (7.7)/31.1 (9.0)b Range: 18–50 | Hospitalized for DSM-IV alcohol dependence. Excluded if taking any medication for medical or psychiatric problems | Five weeks after admission | Average 19.4 drinks per day for 18.6 years | Age of onset of drinking: 19.6 | Excluded if current diagnosis of dependence (except tobacco) | Whole brain grey matter | Reduced grey matter volume in: lateral PFC; (including the right dorsolateral and inferolateral PFC); medial frontal cortex (including the dorsal anterior cingulate gyrus and medial and lateral superior frontal gyrus); the middle frontal gyrus; a posterior region centred on the parietal–occipital sulcus, overlapping the precuneus, cuneus, and posterior cingulate regions |
When calculable, effect sizes reported as Cohen's d.
AD, alcohol dependence; DTs, delirium tremens.
aIf genders have been analysed separately, mean age is displayed separately for each gender. Otherwise mean age is combined.
bSignificant difference between groups.
cUnless specifically stated intracranial volume was controlled for in analyses.
Findings for total grey and white matter cortical volumes were inconsistent. Of the three studies investigating both these measures only one, Agartz et al. (2003) found that both decreased in the alcohol-dependent group (P = 0.007, d = 0.74 for total grey matter and P = 0.004, d = 0.80 for total white matter). This was the only study to examine white matter volumes in women, the gender in which the white matter reduction was particularly pronounced. Fein et al. (2002) did find reduced total grey matter, but this was non-significant on excluding those over 42. The absence of women from this and the Pfefferbaum et al. (1997) studies may be relevant to their negative findings. White matter volume was not compared, but there was no reduction in total grey matter volume in Fein et al.'s (2010) mixed-gender study.
In contrast, reduced frontal cortex grey matter was more consistently reported. Fein et al. (2002) localized reductions to the dorsolateral and posterior prefrontal regions, Pfefferbaum et al. (1997) to the ‘frontal’ region (comprising the middle and more posterior extents of the frontal lobes) and Rando et al. (2011) to regions including the dorsolateral and inferolateral PFC and dorsal anterior cingulate gyrus. When calculable, in the Pefferbaum et al. study, the effect size was modest (P < 0.05, d = 0.44). The only negative study was Fein et al. (2010); this compared the entire frontal lobe and so may have been insensitive to more localized changes. Corpus callosum area was reduced in the two studies which examined it (Hommer et al., 1996; Agartz et al., 2003), though in the study of Hommer et al. this was only significant in women. That this was a consistent finding only in women again suggests women may be particularly susceptible to the effects of alcohol on white matter. Smaller hippocampal volume was much more contentious. Agartz et al. (1999) did detect this in their earlier study, but reduced volume was not specific to the hippocampi, being proportional to that in the rest of the brain. It was not observed in a subsequent report, which adjusted for intracranial volume (Agartz et al., 2003).
Longitudinal studies
Longitudinal data in young people with AUD are few. Bava et al. (2012) publish the only prospective study examining the effects of alcohol use on adolescent brain development. It does not examine adolescents with a defined AUD, but because of the unique nature of this study we nonetheless discuss it in this section. Bava et al. investigated the effects of mixed alcohol and cannabis use in 92 adolescents (63 males and 29 females) with DTI scans separated by 18 months. The mean age of participants was 18.1 ± 1.2 years at entry, ranging from 16.3 to 20.9. Those with >100 lifetime episodes of alcohol or cannabis use constituted the substance using group, controls having <10 lifetime episodes of cannabis use and 80 lifetime episodes of alcohol use. Exclusion criteria included evidence of maternal drug or alcohol use during pregnancy or any Axis I disorder. At baseline, substance users and controls had a mean of 225 vs. 17 lifetime episodes of alcohol use and 453 vs. 1 cannabis use episodes, respectively. Between scans, the user and control groups reported a mean of 408 vs. 70 alcohol use episodes and 652 vs. 13 cannabis use episodes, respectively. The proportion of participants fulfilling diagnostic criteria for an AUD at either baseline or follow-up is not recorded; as discussed, this means that this important study does not fulfil the inclusion criteria for this systematic review. The 51 controls were significantly younger than the 41 in the substance using group, had significantly less externalizing problems at baseline and follow-up as well as used significantly less cigarettes and other drugs than the control group. These factors were included as covariates in the regression analysis, which assessed the influence of substance use in the inter-scan interval on regional white matter anisotrophy at Time 2 above and beyond status at Time 1. Using this methodology, greater alcohol use between scans predicted higher mean diffusivity (indicating lower white matter density), in the right and left superior longitudinal fasciculi (P < 0.05 and 0.06, respectively). The former finding remained significant after controlling for the family history of substance use. Marijuana use between scans did not predict diffusion indices at Time 2 in any of the regions examined. The authors discuss that the superior longitudinal fasciculus undergoes microstructural changes throughout adolescence, that alterations in microstructural and functional integrity of this tract have previously been reported in adolescent alcohol abusers, and suggest that these effects reflect alcohol-related neurotoxicity in cortical brain regions important for fronto-parietal-temporal networks.
Using an adult study group, Bartsch et al. (2007) compare brain volume changes over a 6–7-week period in 15 recently detoxified uncomplicated alcoholics (without heavy nicotine use, mean age 41), with 10 normal controls using automated methodology. They report global brain volume increase of nearly 2% on average which was spatially significant around the superior vermis, peri-mesencephalic, peri-ventricular and frontal brain edges. Similar findings have been reported in older individuals, with DTI studies demonstrating white matter recovery, e.g. Alhassoon et al. (2012). These studies confirm regionally distinct morphological capacities for partial brain recovery from toxic insults of chronic alcoholism.
DISCUSSION
This review suggests that alcohol abuse or dependence is associated with brain structural differences, even in under 40s with ‘uncomplicated’ AUDs, although the literature is not large. These differences are similar to those in older individuals, with volume loss in the frontal/prefrontal lobes being most consistently identified in young adults, but may be more subtle. A small number of studies suggest some particular differences in sub-groups. Lower frontal/PFC volume has been reported in female adolescents. Whereas lower PFC volume in adolescent females may be driven by white matter differences (Medina et al., 2008), in young adults reduced PFC grey matter is more consistently found (Pfefferbaum et al., 1997; Fein et al., 2002; Rando et al., 2011). In contrast to females, one adolescent study reports increased total PFC and PFC white matter volumes in adolescent males (Medina et al., 2008).
Though hippocampal volume reduction has been reported in older alcoholics (even in the absence of amnesia; Sullivan and Pfefferbaum, 2005), it was not a consistent finding in young adults. An early study did report it, but volume reduction was proportional to that in the rest of the brain (Agartz et al., 1999). A subsequent study from the same group, controlling for intracranial volume, reported no differences in either sex (Agartz et al., 2003). In contrast, lower (left) hippocampal volume was reported in the two adolescent studies examining it (De Bellis et al., 2000; Nagel et al., 2005), with large effect sizes (Cohen's d > 0.9), although this finding did fall just short of significance in the De Bellis et al. study when individuals with PTSD were excluded. The influence of confounders will be discussed further below, but these data do suggest that whereas hippocampal volume reduction is observed in adolescents and older adults with AUD, it is less consistently found in young adults.
In contrast with frontal lobe differences in (female) adolescents being apparently driven by white matter reduction, the single (predominantly female) study examining adolescent corpus callosal area did not find it reduced in the AUD group. Corpus callosal area was consistently reduced in young adult women with AUD (Hommer et al., 1996; Agartz et al., 2003).
There are as yet no longitudinal studies examining if AUD is associated with progressive macrostructural brain changes in adolescents or young adults. Although it does not examine a population with AUD, there is, however, a single study, i.e. DTI study, demonstrating that poorer white matter integrity in fibre tracts with frontal connections is associated with adolescent alcohol use. This rather contrasts with the single adolescent cross-sectional DTI study included in this review, which reported that FA was increased in specific corpus collosal regions in a predominantly male adolescent group (De Bellis et al., 2008). One possible explanation for this apparent contradiction is that white matter vulnerability to the effects of alcohol is not uniform throughout the brain, and (in keeping with macrostructural imaging findings) it is adolescent PFC white matter and fibre tracts with frontal connections that are particularly susceptible to alcohol effects. Other explanations are of course possible (see below).
In summary, there are both similarities and differences when comparing brain structural abnormalities in adolescents and young adults. Women of both groups exhibit prefrontal volume reduction, with prefrontal white matter reduction particularly pronounced in female adolescents. Adolescents of both genders exhibit lower hippocampal volume, whereas reduced corpus callosal area was only reported in young adults. That any brain structural differences are more consistently found in the adolescents compared with young adults is surprising. The young adult group will have been drinking for longer and have consumed more alcohol, so if brain structural differences reflected a direct exposure-response effect, they would be greater in this group. Indeed, as adult data suggest an interaction between alcohol and age (Pfefferbaum et al., 1992), even greater differences may be expected in the older group. That the opposite is seen suggests that [in the hippocampi and (female) frontal lobes, at least] alcohol may be interacting with maturational processes specific to late adolescence. The single longitudinal imaging study is consistent with this.
There is animal data suggesting adolescent animals are particularly vulnerable to the neurotoxic effects of alcohol (Hiller-Sturmhofel and Swartzwelder, 2004). The frontal–anterior brain regions have been shown to exhibit this vulnerability in rats (Crews et al., 2000), thus equating to the human frontal cortex. This region undergoes the most marked developmental changes in adolescence (Blakemore and Choudhury, 2006), which may explain why, in females at least, it seems particularly associated with brain structural abnormalities in the context of adolescent alcohol use.
Similarly, binge alcohol exposure in non-human primates was associated with a reduction in hippocampal neurogenesis 2 months after last alcohol exposure (Taffe et al., 2010), and adolescent rats demonstrated persistently greater impairments than adults on a hippocampal-dependent learning and memory task after high levels of alcohol exposure (Sircar and Sircar, 2005; Schulteis et al., 2008). This is in keeping with the imaging data outlined above, suggesting a particular vulnerability of the adolescent hippocampus to the effects of alcohol.
Much of the animal data examines models of exposure designed to mimic adolescent binge exposure rather than alcohol dependence. If adolescent AUDs are associated with brain structural abnormalities, this does raise the question of whether adolescent intermittent high-dose alcohol use in the absence of a diagnosis of alcohol abuse or dependence (i.e. binge drinking) is itself associated with brain structural abnormalities. This question is important as this is a very common form of adolescent alcohol use, with, for example, 36% of men aged 16–24 reportedly consuming five or more drinks in a single session at least once a week (Rickards et al., 2004). A full systematic review of adolescent binge drinking imaging data was beyond the scope of this paper. Of note, however, a DTI study of 14 predominantly male binge drinkers with a mean age of 18.1 (range 16–19) reported reduced FA in 18 white matter areas throughout the brain, including the corpus callosum, superior longitudinal fasciculus, corona radiata, internal and external capsules as well as commissural, limbic, brainstem and cortical projection fibres (McQueeny et al., 2009). There were no areas of higher FA. These findings are rather different from the De Bellis et al. (2008) DTI study, which may be attributable to the older age of the former sample. As mentioned in the Results section, the single longitudinal study in this field does suggest that alcohol use during adolescent neurodevelopment may be linked to reductions in white matter quality in association fibre tracts with frontal connections (Bava et al., 2012). Overall, these data do suggest that adolescent alcohol use falling short of a formal AUD diagnosis may indeed be associated with brain structural abnormalities. A more detailed discussion of the potential mechanisms contributing to the brain structural differences observed in AUD individuals is provided by Crews and Nixon (2009) and Alfonso-Loeches and Guerri (2011).
Alternative explanation for findings
The above suggests that brain structural differences are detectable in adolescents and young adults with AUDs, being particularly exhibited in the adolescent hippocampus. All studies are observational and (aside from the single longitudinal DTI study) cross-sectional, however, meaning causation cannot be determined. Furthermore, it is notable that all the young adult studies in which it was examined did not find a dose response relationship between levels of alcohol exposure and structural measures, or if they did it became non-significant on controlling for age (Hommer et al., 1996; Pfefferbaum et al., 1997; Agartz et al., 1999; Fein et al., 2002; Agartz et al., 2003; Fein et al., 2010). A significant negative correlation was seen between measures of alcohol consumption and structure volumes in the De Bellis et al. (2005) adolescent study, but consumption measures were not correlated with structural measures in the two other adolescent studies, which examined the relationship (Nagel et al., 2005; Medina et al., 2008). Non-causative explanations for the association between alcohol exposure and brain structural differences must be considered.
Comorbidity
On reviewing Table 1, it is notable that many individuals also used other substances (predominantly, tobacco and cannabis), and the majority had DSM-IV Axis I diagnoses other than alcohol abuse/dependence. This is, of course, an issue in young adult as well as adolescent studies. It is recognized, however, that comorbidity is a particular problem in adolescents, polysubstance use being common and conduct disorder being particularly prevalent (Armstrong and Costello, 2002).
In keeping with expectations, the De Bellis et al. studies report conduct disorder and depression as prominent Axis I comorbidities. Both are associated with brain structural abnormalities. Specifically, reduced grey matter has been reported in the amygdala, anterior insula and orbitofrontal cortex of adolescents with conduct disorder (Monti et al., 2005; Sterzer et al., 2007; Fairchild et al., 2011). In a study of young women, reduced sub-genual PFC volume has been reported in adolescent-onset depression (Botteron et al., 2002). DTI has revealed altered white matter microstructure in frontolimbic neural pathways in a mixed-gender group of adolescents with major depressive disorder (Cullen et al., 2010).
The studies undertaken by the San Diego group went to considerable lengths to exclude confounders. The only Axis I comorbidity not an exclusion factor was conduct disorder, and smaller left hippocampi in the AUD group persisted after excluding individuals with conduct disorder (Nagel et al., 2005). Even in this carefully conducted study, however, there were still imbalances between cases and controls in lifetime use of cannabis and tobacco, exposures which could of course themselves be associated with brain structural abnormalities. Indeed, it has been reported that, in older treatment-naıve alcoholics, significant grey matter reductions are primarily seen in those who are also chronic smokers (Durazzo et al., 2007). Consequently, it is conceivable that these comorbidities confound the imaging abnormalities seen.
Trait characteristics
Difficult as these issues are to overcome, even harder to address is the possibility trait characteristics associated both with brain structural abnormalities and a predisposition to alcohol use contribute to the findings. As adolescents with AUD are selected on the basis of an early onset disorder, they would be expected to have a greater ‘loading’ of this factor, potentially resulting in both earlier onset of AUD and more pronounced structural abnormalities. Any such ‘third factor’ would be less obvious than those discussed above (and consequently, even harder to control for), but potential candidates would be genetic risk factors, pre-/peri-natal exposures or events, early life experiences or Axis II pathology. The latter is not discussed by either group examining adolescents, but given the high levels of conduct disorder significant comorbidity would be expected (Burket and Myers, 1995). The studies of the San Diego group are, of course, important in trying to minimize the influence of any such confounder. As cases and controls were balanced for the family history of substance use disorders, genetic (and potentially other) factors predisposing to alcohol use should be reasonably balanced in the two groups. This does not, however, exclude the possibility that other trait characteristics associated with elevated risk of AUDs could still explain brain structural differences between the groups; indeed, this is suggested by the greater prevalence of conduct disorder in the alcohol using group.
Longitudinal studies have the potential to reduce the influence of any ‘third factor’. If individuals have already begun using alcohol by the time of recruitment, however, one can still not assume that any changes subsequently seen in association with alcohol use are caused by it. An unidentified confounder could still potentially account for an abnormal trajectory of brain development and predispose to alcohol use. Studies of individuals who are at high risk of AUD (but have not yet consumed alcohol) are crucial in establishing if trait characteristics associated with the risk of AUDs are associated with brain structural abnormalities. Three groups, such as Hill et al. in Pittsburgh, Benegal et al. in Bangalore and Tapert et al. in San Diego, have examined if a familial propensity to alcohol problems is associated with brain structural abnormalities in individuals with no/minimal alcohol exposure. Hill et al. (2001) reported that, after controlling for current alcohol consumption (18.2% were already alcohol- or drug- dependent at the time of assessment), male adolescents at high risk of alcohol problems on the basis of family history had reduced the volume of both the right amygdala and right orbitofrontal cortex (Hill et al., 2009). In a subsequent expanded sample, increased total and grey matter cerebellar volume was confirmed in the high-risk group, even after excluding those with alcohol or drug use disorders (Hill et al., 2011). Benegal et al. (2006) focussed on a slightly younger, alcohol naïve, high-risk group (mean age 15). In contrast to Hill et al., they reported decreased cerebellar volume. They also reported bilateral amygdala and hippocampal reduction. Their voxel-based morphometry analysis reported grey matter deficits in many additional regions, including the thalami, superior frontal gyri and cingulate gyri. A subsequent study reported reduced total corpus callosal area, localizing this to the genu and isthmus (Venkatasubramanian et al., 2007). This does not seem compatible with the suggestion of De Bellis et al. that there is accelerated myelination in corpus callosal regions in individuals at high risk of AUD. Finally, Wetherill et al. (2011) used DTI to demonstrate that substance-naïve youth with a family history of alcohol dependence (aged 12–14) did not show differences in white matter architecture within tracts sub-serving fronto-parietal circuitry. They also replicated Hill et al.'s finding that hippocampal volumes were not reduced in adolescents with a family history of alcoholism, and actually suggested that males with this history had increased left hippocampal volume (Hanson et al., 2010). These high-risk studies, therefore, suggest that abnormalities observed in the amygdala, thalamus, corpus callosum, cingulate and regions of the prefrontal lobe in young alcohol abusers may be partly due to pre-morbid factors. In contrast, the lack of apparent reductions in hippocampal volume in those at high risk suggests that the reduced hippocampal volume in adolescents with AUD may be a direct consequence of alcohol exposure, potentially due to a particular vulnerability to the effects of alcohol in adolescents. This may be explained by alcohol exposure interacting with adolescent brain development and leading to the derangement of maturational processes.
Variability in study methods
It is possible that factors such as recruitment methods, study inclusion criteria, sample size and technical differences in image processing and analysis could result in the contrasting findings in adolescents and young adults. Considering recruitment methods, it has been demonstrated that treated and treatment naïve alcoholics differ with regard to factors, such as historic levels of alcohol use (Fein and Landman, 2005) and some imaging measures (Gazdzinski et al., 2008). Reassuringly, however, in both age groups, the included studies were a mixture those which recruited from the community and treatment services, lessening this possibility. Of greater potential importance is the fact that the scanners used in the (all post-2000) adolescent studies were higher resolution, which could potentially increase the likelihood of detecting significant differences between the AUD subjects and controls in this age group. While this possibility cannot be fully dismissed, it is reassuring that the earlier young adult studies (which will have used lower resolution scanners) seem to have been no less likely to report positive findings than the more recent ones. Finally, differing methods of image analysis in the adolescent and young adult groups could potentially have meant that positive findings were more likely in the former. It is the case, however, that in both age groups a comparable mix of automated and semi-automated image analyses approaches were employed.
Other limitations
The choice of 21 as the age to distinguish between adolescent is fairly arbitrary, and we acknowledge that brain maturation is known to continue well into the 20s (Sowell et al., 1999). In reality, however, studies were not identified focusing on individuals in their early 20s, so this should not impact on the findings of the review.
Future directions
Further clarification of the possibility that adolescents are particularly susceptible to the consequences of alcohol use necessitates more longitudinal studies. Ideally, these would identify individuals at risk of alcohol dependence prior to first alcohol use and follow them up with sequential scans over the period during which alcohol use would be expected to begin. Definitively, establishing if adolescents are particularly susceptible to the brain structural consequences of alcohol use would require inclusion of an older comparator group. Such studies would have the potential to build on this review, yielding further insights into the brain structural consequences of adolescent alcohol use. Unfortunately, no longitudinal imaging study currently has addressed macrostructural brain characteristics. As well as their DTI study, however, Tapert et al.'s group have also published a longitudinal functional imaging study, which recruited adolescents with minimal prior alcohol exposure (Squeglia et al., 2012). They found that lower baseline frontal and parietal activation predicted transition to heavy drinking, with activation increasing at the second scan in those who initiated drinking though it decreased in those who did not. This is interpreted as suggesting that pre-existing executive dysfunction may predispose to heavy drinking with the onset of drinking leading to interference with efficient information processing. Further longitudinal studies such as this have the potential to increase our understanding of why some adolescents are particularly vulnerable to initiating substance misuse and to guide the development of protective interventions.
Conflict of interest statement. None declared.