-
PDF
- Split View
-
Views
-
Cite
Cite
Frédérique Souazé, Véronique Viardot-Foucault, Nicolas Roullet, Mireille Toy-Miou-Leong, Anne Gompel, Erik Bruyneel, Eva Comperat, Maree C Faux, Marc Mareel, William Rostène, Jean-François Fléjou, Christian Gespach, Patricia Forgez, Neurotensin receptor 1 gene activation by the Tcf/β-catenin pathway is an early event in human colonic adenomas, Carcinogenesis, Volume 27, Issue 4, April 2006, Pages 708–716, https://doi.org/10.1093/carcin/bgi269
Close - Share Icon Share
Abstract
Alterations in the Wnt /APC (adenomatous polyposis coli) signalling pathway, resulting in β-catenin/T cell factor (Tcf)-dependent transcriptional gene activation, are frequently detected in familial and sporadic colon cancers. The neuropeptide neurotensin (NT) is widely distributed in the gastrointestinal tract. Its proliferative and survival effects are mediated by a G-protein coupled receptor, the NT1 receptor. NT1 receptor is not expressed in normal colon epithelial cells, but is over expressed in a number of cancer cells and tissues suggesting a link to the outgrowth of human colon cancer. Our results demonstrate that the upregulation of NT1 receptor occurring in colon cancer is the result of Wnt /APC signalling pathway activation. We first established the functionality of the Tcf response element within the NT1 receptor promoter. Consequently, we observed the activation of NT1 receptor gene by agents causing β-catenin cytosolic accumulation, as well as a strong decline of endogenous receptor when wt-APC was restored. At the cellular level, the re-establishment of wt-APC phenotype resulted in the impaired functionality of NT1 receptor, like the breakdown in NT-induced intracellular calcium mobilization and the loss of NT pro-invasive effect. We corroborated the Wnt /APC signalling pathway on the NT1 receptor promoter activation with human colon carcinogenesis, and showed that NT1 receptor gene activation was perfectly correlated with nuclear or cytoplasmic β-catenin localization while NT1 receptor was absent when β-catenin was localized at the cell–cell junction in early adenomas of patients with familial adenomatous polyposis, hereditary non-polyposis colorectal cancer and loss of heterozygosity tumours. In this report we establish a novel link in vitro between the Tcf/β-catenin pathway and NT1 receptor promoter activation.
Introduction
Among the signalling elements involved in tumour progression, oncogenic pathways generated by G-protein coupled receptors (GPCR) are increasingly implicated ( 1 ). The link between GPCR signalling and cancer progression has been recently exemplified by the activation of the Rho family small GTPases through the G-protein subunits Gα12/α13 and Gαq/11 ( 2 ). Rho-like GTPases regulate the extracellular stimuli-mediated signalling pathways that control gene expression, actin cytoskeletal organization, cell cycle progression, survival and invasiveness ( 3 – 5 ).
The tridecapeptide neurotensin (NT) acts as a paracrine and endocrine modulator of a variety of gut functions. NT effects are mainly mediated by the high-affinity neurotensin receptor (NT1 receptor), a seven transmembrane GPCR coupled to Gαq/11 ( 6 ), stimulating RhoGTPases (RhoA, Rac1, Cdc42) and focal adhesion kinase (FAK) ( 7 , 8 ). In the gastrointestinal tract, NT is synthesized by the endocrine N cells and released in the blood circulation ( 9 ). This release is enhanced by nutritional factors, such as fat-rich meals ( 10 ), which has been suggested to be associated with colorectal cancer incidence data indicates incidence in the Western population ( 11 ). Abundance of data indicates that NT is implicated as a trophic factor in colon, prostate, pancreatic and lung cancer ( 12 ). Moreover, recent studies have demonstrated the ability of endogenous NT to promote the growth of human lung and colon cancer cells xenografted in mice ( 13 , 14 ).
Epithelial crypts isolated from normal human adult colon lack the NT1 receptor, whereas it is overexpressed in numerous adenocarcinoma cell lines ( 15 ), suggesting that this GPCR is induced during colonic neoplastic progression. In support of this hypothesis, analysis of the regulatory sequences in the NT1 receptor gene revealed the presence of a consensus T cell factor (Tcf) binding site potentially linking the activation of the NT1 receptor and APC (adenomatous polyposis coli)/β-catenin pathway. Constitutive activation of the APC/β-catenin pathway occurs through alterations in the tumour suppressor gene APC and stabilization of β-catenin in sporadic and familial colorectal tumours, including familial adenomatous polyposis (FAP) and hereditary non-polyposis colorectal cancer (HNPCC) ( 16 ). In normal cells, the multimolecular protein complex comprising APC, glycogen synthase kinase (GSK)-3-β and axin/conductin triggers the degradation of the cytosolic β-catenin via the ubiquitin-proteasome pathway. In colon cancer cells, the deactivation of β-catenin degradation pathway by Wingless/Wnt signalling, or by mutations in APC or β-catenin, causes aberrant cytosolic and nuclear β-catenin accumulation ( 17 ). The ensuing association between nuclear β-catenin association and lymphoid enhancer factor (LEF)/Tcf transcription factors causes the abnormal expression of oncogenes involved in cell cycle regulation, cellular differentiation and cellular invasion [for a review see van Es et al . ( 18 )].
In this study we establish the ability of Wnt/APC signalling and β-catenin/Tcf complex to induce the activation of the NT1 receptor gene through the consensus Tcf–DNA binding site localized within the NT1 receptor gene promoter. We observe the NT1 receptor expression in human colonic adenoma suggesting that NT/NT1 receptor system may be implicated early in the progression of human colorectal cancers.
Materials and methods
Cell culture and transfections
HEK 293T, HT-29 and Cos-7 cells were grown in Dulbeco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 2 mM glutamine. HT-29 APC containing a zinc-inducible wild-type (wt)- APC transgene and the control cell line, HT-29 β-Gal, containing an analogous inducible LacZ gene, were kindly provided by Dr Vogelstein (Johns Hopkins University, Baltimore, MD). Cells were grown in McCoy's 5A media supplemented with 10% FCS and 0.6 mg/ml of hygromycin. The transgene was induced by 150 μM ZnCl 2 for 24–48 h. Parental SW480 cells and (pcDNA3 APC full-length) stably transfected SW480 APC cells were grown in RPMI 1640 (Invitrogen, USA) supplemented with 1.08% 10-2 thioglycerol, 100 U/ml insulin, 50 mg/ml hydrocortisone, 10% FCS and 1% penicillin/streptomycin. Wt- APC transgene expression was maintained with 1.5 mg/ml geneticin ( 19 ). The human breast epithelial cells (HBEC) were plated and grown as described previously by Gompel et al . ( 20 ). Breast tissue was obtained from women undergoing reduction mammoplasty. The patients had no history of breast disease and pathological study showed only normal breast tissue. Sampling of the tissues was performed according to the French regulation on clinical experimentation. All media were supplemented with 10% FCS and 2 mM glutamine. Using lipofectamine reagent (Invitrogen, France), HEK 293T cells were transfected with 10 μg of β-catenin (pCI-neo-β-catenin), Wnt-2 (pLNC-Wnt2-HA) or Wnt-7b (pUSE-Wnt7b-HA) (Upstate Biotechnology, USA) expression vectors. Cells were harvested 48 h after transfection.
A 1554 bp sequence of the 5′ end of the NT1 receptor gene (position 71373–72926 in the human DNA sequence, AL357033), was inserted upstream of the coding region for the luciferase reporter gene in the pUT103 vector (CAYLA, France). Using the calcium phosphate co-precipitation method for 16 h at 37°C ( 21 ), Cos-7 cells were co-transfected with 2 μg of the NT1 receptor promoter luciferase reporter construct, 1 μg pCI-NEO-β-catenin and/or 0.5 μg pcDNAI-hTcf4 expression vector ( 22 ). The pRSV-β-gal (400 ng) was co-transfected as a transfection efficiency reference and the β-galactosidase assay was performed with Galacto-star TM (Applied Biosystems, France). The transfection solution of each condition was filled up to 4 μg with pDNA3-CAT plasmid DNA (InVitrogen, France). Each luciferase activity was divided by β-galactosidase activity and protein content. The consensus Tcf site CTTTGATGA at the position (−1188 and −1180 bp) within the NT1 receptor promoter was mutated to the CTTTG GC GA sequence using the QuickChange Site-directed Mutagenesis Kit (Stratagene, USA).
Gel shift assay
Cos-7 nuclear protein extracts were isolated as reported previously ( 23 ). 20mer synthetic double-stranded oligonucleotides containing the native (5′-CCAGACTTTGATGAAACCA-3′) or the mutated Tcf consensus site (5′-CCAGACTTTG GC GAAACCA-3′) of the human NT1 receptor promoter were end-labelled with T 4 polynucleotide kinase and [γ- 32 P]ATP (3000 Ci/mmol; Amersham Pharmacia Biotech, Saclay, France), as described previously ( 24 ). Binding reactions were performed in 20 μl containing 25 mM HEPES (pH 7.2), 150 mM KCl, 5 mM DTT, 10% glycerol, 1 μg of poly(dI–dC), 20 μg of fresh nuclear extract and 10 5 c.p.m. of labelled probe, for 20 min at room temperature. For competition experiments, nuclear extracts were pretreated for 10 min with 100-fold excess of unlabelled competitors. Antibody super-shift assays were performed by adding 2 μg of mouse monoclonal antibody directed against Tcf-4 (Upstate biotechnology, USA) at the mid-reaction. Control and transfected samples were performed in the same experiment. DNA–protein complexes were resolved on 5% non-denaturing polyacrylamide gels at 150 V in 0.25× TBE buffer for 90 min at 22°C. The gels were dried and autoradiographed using intensifying screens.
Total cellular protein extract preparation
Wnt-2 or Wnt-7b transfected HEK-293T cells were washed twice and resuspended in Dulbecco's phosphate-buffered saline (PBS). After two subsequent centrifugations at 9000 g for 10 min, the cellular pellet was resuspended in 200 μl of Dulbecco's PBS supplemented by 5.7 mM PMSF (phenylmethylsulfonyl fluoride; Sigma, USA) and aprotinin 2.25 mg/ml (Boeringher, Germany) and sonicated for 15 s. After incubation for 30 min at 4°C, the suspension was centrifuged at 4°C and 15 000× g , for 20 min. Protein content in the supernatant was assayed using the BCA method (Pierce Biotechnology, USA).
β-Catenin immunocytochemistry
HBEC cells were plated on 24-well dishes and treated or not with 20 mM LiCl or 20 μg protein extract from HEK 293T cells transfected with pcDNA3 or Wnt-7b expression vector, for 6 h. Briefly, cells were fixed 2 min by acetone/methanol (v/v) solution and β-catenin labelling was performed with rabbit β-catenin polyclonal antibody (1/500) (c2206, Sigma, France) and revealed with secondary Texas-Red anti-rabbit antibody (1/200).
RNA extraction and RT–PCR
The protocols for total RNA extraction, reverse-transcription reaction (RT), and PCR are documented in Souazé et al . ( 24 ). RT was performed on 2 μg of total RNA using a specific NT-1 receptor primer (5′-GCTGACGTAGAAGAG-3′) or 50 pmol of oligo dT and oligo dN. The PCR amplification was performed on a 1:5 (v/v) of the RT reaction using 25 pmol of each primer 5′-CGTGGAGCTGTACAACTTCA-3′ and 5′-CAGCCAGCAGACCACAAAGG-3′ and 1 U of Taq polymerase (Applied Biosystems, France). The amplification profile consisted of denaturation at 94°C for 30 s, annealing at 57°C for 45 s and extension at 72°C for 45 s. The 32 cycles of PCR were preceded by denaturation at 95°C for 5 min and were followed by a final extension at 72°C for 10 min. Amplification was performed in a DNA thermal cycler 9700 (Perkin Elmer Applied Biosystems, Courtaboeuf, France). PCR products were electrophoresed on 1% agarose gels. The bands were visualized with ethidium bromide.
Western-blot
For APC protein detection, HT-29 cells were lysed as described by Su et al . ( 25 ) and western blot procedure was performed on 100 μg protein extract as described by the mouse monoclonal APC (Ab-1) antibody manufacturer (Oncogene Research Products, USA). NT-1 receptor protein detection was performed on 20–80 μg of HT-29 or SW480 membrane preparation ( 26 ), using a goat polyclonal antibody against human NT1 receptor (C-20) at the dilution (1/500) (Tebu, France). The antigen–antibody complex was visualized with horseradish peroxidase-conjugated, goat anti-mouse or rabbit anti-mouse antibodies and the ECL system (Amersham, France).
Binding studies
Cell membrane preparations were obtained from HT-29 β-gal, HT-29 APC, parental SW 480 and SW 480 APC + cells using the procedure of Boudin et al . ( 26 ). Cells were washed three times with cold PBS. Radioligand binding studies were performed as followed: 60 μg of protein were incubated with 0.1 nM 125 I-NT in a final volume of 250 μl of buffer A [50 mM Tris (pH 7.4), 0.2% BSA and 0.8 mM 1.10-orthophenantroline and 1.6 mM MgCl 2 ] for 1 h at room temperature. Non-specific binding was measured in the presence of 1 μM unlabelled NT. Binding assays were terminated by addition of ice-cold 50 mM Tris–HCl (pH 7.4), followed by filtration through glass-microfiber filters (GF/B, Whatman) preincubated in 0.2% polyethylenimine. After washing three times with 5 ml ice-cold buffer, the radioactivity retained on the filters was counted in a γ-counter (Perkin Elmer, Wallac model 1470 Wizard, France).
Quantification of intracellular calcium levels
Changes in intracellular Ca 2+ concentration in response to 100 nM JMV 449 and 1 mM ATP were measured on HT-29 β-gal or HT-29 APC cell lines treated or not with 150 μM ZnCl 2 for 36 h. Cells cultured on 22 mm polylysine-coated glass coverslips, were loaded with 4 μM Fura-2/acetoxymethyl ester (Molecular Probes, InVitrogen, France) in serum-free medium for 30 min and washed with serum-free medium at 37°C for 30 min. The medium was then replaced with 360 μl recording buffer (8 mM Na 2 HPO 4 , 1.5 mM KHPO 4 , 138 mM NaCl, 2.7 mM KCl, 20 mM HEPES, 1.3 mM CaCl 2 , 0.8 mM MgCl 2 and 5 mM glucose) and coverslips were mounted into a 37°C recording chamber installed on the stage of an inverted epifluorecence microscope (Nikon Diaphot 300, Japan). For Fura-2 dual excitation, the beam of light from a high-pressure xenon bulb set was passed through a 340/380 nm shutter/filter wheel (Applied Imaging, Visitech, UK) at 0.5 Hz. The emitted fluorescence was collected by a 40× oil objective and detected at 510 nm. Fluorescence signals were digitized and stored on disk using Quanticell 700 (Applied Imaging). Free intracellular calcium concentration ([Ca 2+ ] i ) was monitored by using Fura-2 ratio imaging with the parameters: R = F 340 /F 380, Rmin = 0.3, Rmax = 3, ß = 3.42 and KD = 220 nM ( 27 ).
Collagen invasion assays
Details of the procedure was described by Brake et al . ( 28 ). Briefly, Petri dishes were filled with 1.35 ml of neutralized type I collagen and incubated overnight at 37°C to allow gelling. HT-29 and SW480 cells were harvested and isolated using Moscona buffer and trypsin/EDTA, then seeded on top of the collagen gels at the density of 0.33 × 10 6 cells per dish. Cells were cultured for 24 h at the indicated temperature in the presence or absence of the NT agonists alone, or with SR 48692 (2-[(1-(7-chloro-4-quinolinyl)-5-(2,6-dimethoxyphenyl)pyrazol-3-yl)carbonylamino]tricyclo(3.3.1.1. 3.7 )decan-2-carboxylic acid) or NT neutralizing antibody (Monosan, The Netherlands). Invasive and superficial cells were counted in 12 fields of 0.157 mm 2 . The invasion index was calculated as the percentage of cells invading the gel divided by the total number of cells ( 28 ).
Patient specimens for immunohistochemistry and RT–PCR
For immunohistochemistry, surgical specimens were retrieved from the files of the Department of Pathology, Saint-Antoine Hospital. Cases were classified in four groups (FAP, n = 8; HNPCC, n = 8; sporadic with loss of heterozygosity (LOH), ( n = 10); and sporadic with microsattelite instability (MSI), n = 4) according to standard criteria, as published previously ( 29 ). Formalin-fixed, paraffin-embedded tissue sections were de-paraffinized in xylene for 10 min. Tissue sections were then gradually hydrated through graded alcohol washes and rinsed with water. After hydration, tissue sections were incubated in 3% hydrogen peroxide for 5 min to quench endogenous peroxidase activity, and rinsed three times for 5 min with TBS containing 0.01% Tween-20 and 0.06% casein. NT1 receptor immunoreactivity was detected using a goat polyclonal antibody (1:100) directed against the C-terminus of the human receptor (Santa Cruz, USA). NT1 receptor immunohistochemistry specificity was checked by the omission of primary antibody and displacement with neutralizing peptide (Santa Cruz). For β-catenin, antigen retrieval consisting in microwave processing at 750 and 150 W for 15 min each and pressure-cooking in 0.01 M citrate buffer (pH 6.0) was applied. The slides were incubated with a monoclonal antibody against β-catenin at 1:250 (E-5, Santa Cruz Biotechnology, directed against amino acids 680–781 mapping at the C-terminus of β-catenin of human origin) ( 30 ). All slides were rinsed three times with Tris-buffered saline; sections were incubated with biotinylated secondary antibody (1:200) (Vector, USA), for 30 min at room temperature. The antigen–antibody complex was revealed with avidin–biotin–peroxidase complex for 30 min according to the manufacturer's instructions for the Vectastain® ABC Kit (Vector). Staining was done for 5 min with diamino-benzidine tetrahydrochloride (DAB) (Sigma). All slides were counterstained with hematoxylin.
Results
NT1 receptor promoter is a target of the Tcf/β-catenin complex
It was shown previously that the Tcf/β-catenin complex activates target genes implicated in various types of cancer including colon cancer ( 18 ). NT1 receptor was shown previously to be overexpressed in numerous cancers derived from epithelial cells ( 12 , 15 ). The analysis of the NT1 receptor promoter sequence reveals a consensus Tcf-DNA binding site, at the position −1188 bp relative to the AUG translation initiation site ( 31 ). Accordingly, we investigated the potential of Tcf4/β-catenin complex to stimulate NT1 receptor promoter at this Tcf site. As shown in Figure 1A , we observed a 4 to 5-fold transcriptional activation, relative to the control, when β-catenin and hTcf4 were co-transfected, in Cos 7 cells, with NT1 receptor promoter linked to the luciferase reporter gene. A similar response was seen when hTcf4 was co-transfected with a stable β-catenin mutant ΔN89, a β-catenin form insensitive to ubiquitination (data not shown). This activation was abolished when NT1 receptor Tcf binding site was mutated ( Figure 1A ). We confirmed the physical implication of the NT1 receptor Tcf binding site in Tcf/βcat transcriptional responsiveness using a gel shift assay. A retained band was observed when the native Tcf element was exposed to nuclear extracts obtained from Cos-7 cells transfected with hTcf4 and β-catenin ( Figure 1B , lanes 2 and 6) compared to the non-transfected cells ( Figure 1B , lanes 1, 3 and 5). The presence of hTcf4 in the retained band was confirmed by the super shifted band observed when specific antibody directed against hTcf4 was added to the reaction ( Figure 1B , lane 4).
NT1 receptor is a Tcf-4/β-catenin target gene. ( A ) Luciferase reporter activity of NT1 receptor promoter. Cos-7 cells were transiently transfected with wild-type (wt), or mutated promoter-luciferase reporter constructs, or empty vector control plasmid, together with β-catenin and/or hTcf4 expression vectors. The results were expressed as fold induction, and in each measurement luciferase activity was divided by β-galactosidase activity and protein content. Results are the mean of four independent experiments. ( B ) Electrophoretic mobility shift assay of mock (lanes 1, 3 and 5) or Tcf/β-catenin (lanes 2, 4 and 6–9) transfected Cos-7 nuclear extracts, with [γ- 32 P]ATP-labelled probes. Lanes 1–8, wild-type probe (Wt Tcf); lane 9, mutated probe in the putative Tcf-4 DNA binding site (Mut Tcf). In lanes 3 and 4, the Tcf-4 antibody was added to the nuclear lysates. Lanes 7 and 8 show competition with a 100-fold excess of wild-type and mutated cold probe, respectively. This is a representative experiment of three independent experiments. ( C ) Accumulation of endogenous NT1 receptor transcripts detected by RT–PCR following over-expression β-catenin in HEK 293T cells, or treatment of normal HBEC with Wnt factors or LiCl. HBEC were incubated for 48 h with cellular extract from mock or Wnt-2 or Wnt-7b-transfected HEK 293T cells. This is a representative experiment of three independent experiments. ( D ) β-catenin immunocytochemistry (red) and nuclear DAPI (blue) staining of HBEC control, or treated, for 6 h, with cellular extract from pcDNA3 or Wnt-7b-transfected HEK-293T cells, or 20 mM LiCl.
We confirmed the specificity of the retained band by competition experiments. The retained band disappeared when an excess of cold wild-type probe was added in the reaction mixture ( Figure 1B , lane 7), whereas the band remained when the mutated probe was used ( Figure 1B , lane 8). No retained band could be detected using labelled mutated probe ( Figure 1B , lane 9). These data suggest that NT1 receptor promoter is a target of Tcf/β-catenin complex via its consensus Tcf-DNA binding site.
This hypothesis was substantiated with the activation of endogenous NT1 receptor gene in the Tcf-positive cell line, HEK 293T, transfected with wt β-catenin. An increase in NT1 receptor transcript levels is shown in Figure 1C , left. We confirmed this result in normal HBEC in primary culture, which do not express NT1 receptor. To impair β-catenin phosphorylation and degradation, HBEC cells were treated, with Wnt2 or Wnt7b HEK 293T transfected cellular extracts, or with LiCl. We observed that Wnt factors or LiCl induced NT1 receptor mRNA expression without GAPDH expression modification ( Figure 1C , middle and right). To confirm the effect of LiCl and Wnt factors on β-catenin cellular localization, β-catenin immunocytochemistry was performed on HBEC. In control cells or cells treated with pcDNA3 transfected HEK 293T cellular extracts, for 6 h, β-catenin labelling on HBEC is mainly localized at the cell junction ( Figure 1D ). In contrast, as shown by the overlay between the DAPI staining (blue) and the β-catenin staining (red), in HBEC treated with Wnt-7b HEK 293T transfected cellular extract or 20 mM LiCl, for 6 h, β-catenin is localized in the nuclear region ( Figure 1D ). These results support the hypothesis that NT1 receptor gene is a downstream target of the Wnt/β-catenin signalling pathway.
Restoration of full-length APC protein expression in colon cancer cell lines decreases NT-1 receptor expression and function
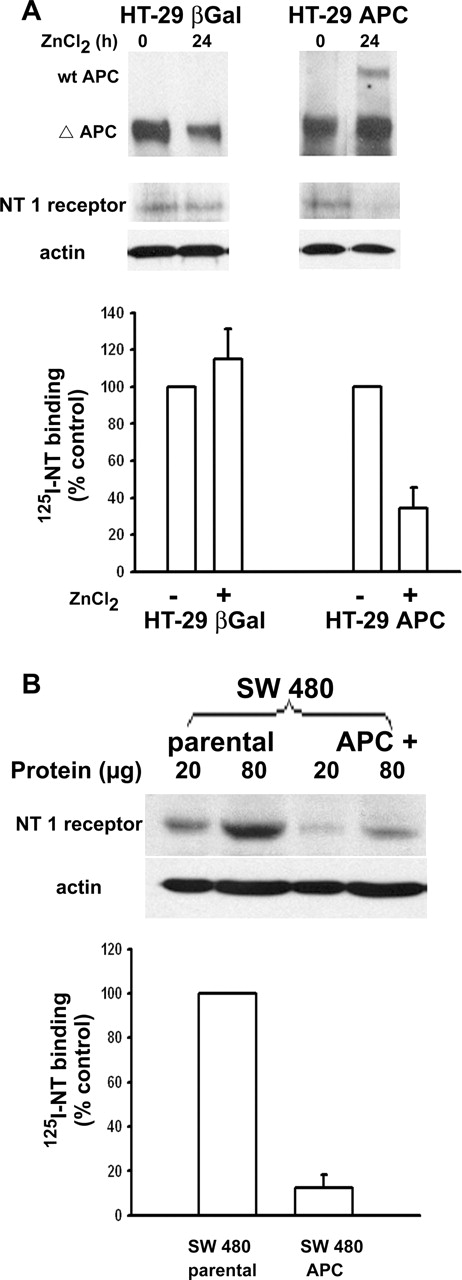
In order to establish the relationship between β-catenin stabilization and NT1 receptor expression in APC-deficient colon cancer cells, we took advantage of two colon cancer cell lines in which full-length APC protein could be induced by zinc (HT-29 APC) or has been restored (SW480 APC). We first compared wt-APC and NT1 receptor protein expression in control and ZnCl 2 -treated HT-29 APC cells. We showed that concomitantly to APC full-length expression, the level of NT1 receptor protein, visualized by western blot or immunocytochemistry, decreases in HT-29 APC, whereas the same treatment did not modify NT1 receptor immunoreactivity in control cells, HT-29 β-gal cells ( Figure 2A ). This diminution in NT1 receptor expression is accompanied by a 70% decrease in 125 I-NT specific binding in the HT-29 APC cells treated with ZnCl 2 for 24 h compared with HT-29 β-gal. As expected, the non-treated HT-29 β-gal and HT-29 APC cells exhibited a high specific binding (95%) with the similar high level of specific binding 98 ± 8 and 154 ± 29 fmol/mg protein, respectively. In SW480 APC cells expressing full-length APC protein, NT1 receptor expression and 125 I-NT specific binding are also reduced compared to parental SW 480 cells ( Figure 2B ).
wt-APC expression repress NT1 receptor. ( A ) Western-blot analysis was performed in HT-29 APC and HT-29 β-gal cell lines after 150 μM ZnCl 2 treatment for 24 h, using (top) a monoclonal antibody detecting full-length (300 kDa) and truncated forms of APC, or (middle) a polyclonal antibody directed against human NT-1 receptor (42 kDa). Both western blots were performed on protein extracts provided from the same experiments. PVDF membrane used for NT1 receptor detection was re-probed with a β-actin antibody as loading control (bottom). 125I-NT binding experiments were performed on 80 μg of membrane extracts from HT-29 APC or HT-29 β-gal treated or not by 150 μM ZnCl 2 for 24 h (bottom). ( B ) Western-blot analysis of NT1 receptor in parental and APC + SW480 cells (top), PVDF membrane was re-probed with a β-actin antibody as loading control (bottom). 125I-NT binding experiments were performed on 80 μg of membrane extracts from parental and APC + SW480 cells. These results are representative of at least three independent experiments.
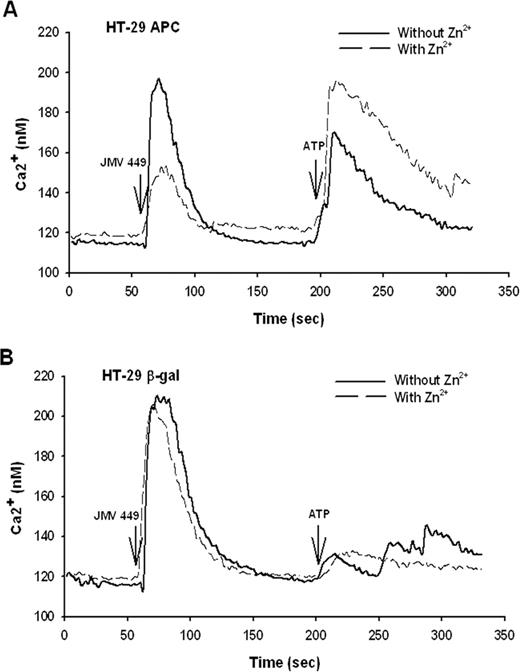
We then studied if the restoration of the full-length APC protein expression impaired the functionality of NT1 receptor. First, we examined Ca 2+ mobilization induced by NT agonist, the JMV 449, in HT-29 β-gal or in HT-29 APC cells, control or treated under the condition allowing the expression of wt-APC ( Figure 2A ). Induction of wt-APC, by ZnCl 2 , for 36 h, markedly decreases the Ca 2+ mobilization in HT-29 APC compared to non-treated cells ( Figure 3A ). The calculation of the area under the peak induced by NT indicates a 60% decrease when wt-APC is expressed. In contrast, ZnCl 2 treatment had no effect on Ca 2+ mobilization in HT-29 β-gal cells ( Figure 3B ). We verified the ability for the HT-29 APC cells treated with Zn 2+ to respond to another stimulus. The ATP stimulation following the JMV 449 stimulation shows an intense response in the HT-29 APC ZnCl 2 treated cells, indicating the healthiness of the cells ( Figure 3A ). In each case after a strong Ca 2+ mobilization response induced by JMV 449, we observed that the Ca 2+ mobilization induced by ATP was much weaker ( Figure 3A and B ). It is most likely because the Ca 2+ storage vesicles were not restored because of the short period of time between the two stimulations.
Effect of wt-APC protein expression on NT-induced intracellular calcium signalling. Intracellular Ca 2+ was monitored in HT-29 APC or HT-29 β-gal cells treated or not with 150 μM ZnCl 2 , for 36 h, loaded with Fura-2/acetoxymethyl ester and stimulated with 100 nM of NT agonist, JMV 449, followed by 1 mM ATP as a positive control (see Materials and methods section). ( A ) JMV 449-induced intracellular Ca 2+ changes were compared in normal and ZnCl 2 treated HT-29 APC cells to induce wt- APC gene expression (see Figure 2 ). The plots represent the average of 85 and 55 cells for control or ZnCl 2 treated cells, respectively. ( B ) JMV 449-induced intracellular Ca 2+ changes of HT-29 β-gal cells were compared in control or ZnCl 2 treated cells. The plots represent the average of 52 and 35 cells for control or ZnCl 2 treated cells, respectively.
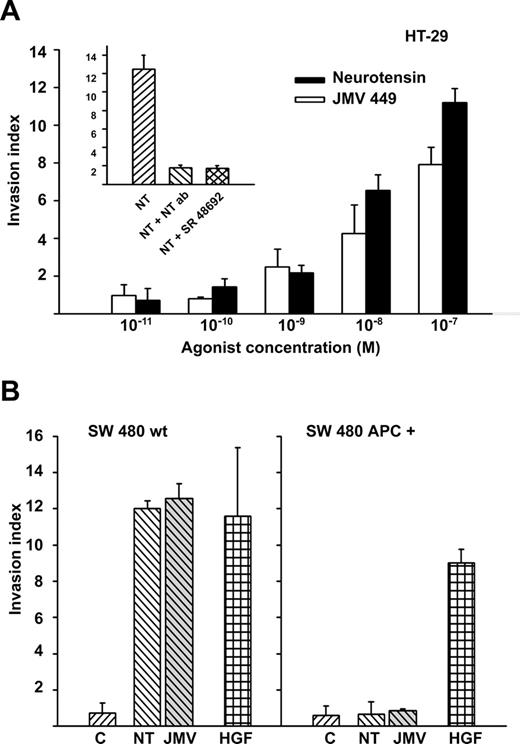
Finally, we substantiate the function of APC signalling on NT pro-invasive function in two human colon adenocarcinoma cell lines expressing the NT1 receptor, HT-29 and SW480 cells. As shown in Figure 4A and B , both NT and JMV449 induced the invasive phenotype in parental HT29 and SW480 cells. As expected, the effect observed with 100 nM NT is reversed by a co-treatment with (1/50) dilution of an antibody directed against NT, or with 1 μM of the NT1 receptor antagonist, the SR48692. As control experiments, we verified that SR48692 and NT antibody had no effect on HT-29 cellular invasion induced by leptin and the trefoil peptide TFF3 (data not shown). As shown in Figure 4B , restoration of full-length APC protein in SW480 cells resulted in a loss of NT-induced invasive phenotype, whereas these cells were still responsive to hepatocyte growth factor (HGF) in the collagen invasion assay.
Effect of wt-APC on pro-invasive activity of neurotensin. ( A ) NT or JMV 449 dose-dependent induction of cellular invasion in Type I collagen by parental HT-29 cells. In insert, cellular invasion blockade induced by 100 nM NT, by NT blocking antibody or NT1 receptor antagonist, SR48692. ( B ) NT or JMV449 (100 nM) induced invasion in Type I collagen was compared in parental SW480 cells (SW480 wt) and SW480 cells expressing full-length APC protein (SW480 APC + ). HGF was used as positive control.
The compilation of these data demonstrates that in vitro NT1 receptor is a target gene of Wnt/APC pathway, and restoration of normal β-catenin degradation pathway by restoration of wt-APC impaired NT1 receptor expression, its signalling and function.
Abnormal expression of NT1 receptor correlates with cytosolic or nuclear β-catenin accumulation in colonic adenomas and adjacent normal mucosa
In order to confirm the link between the NT1 receptor expression and the patterns of β-catenin localization, we performed NT1 receptor and β-catenin immunohistochemistry on different surgical tissue sections from patients with colon cancer syndrome classified according to familial criteria (FAP and HNPCC), or from sporadic colorectal carcinomas (LOH, characterized by aneuploidy, allelic losses and phenotypic MSI). We compared NT1 receptor labelling and β-catenin staining performed on adjacent tissue section from the same patients. As it was described previously ( 32 ), heterogeneous cellular β-catenin staining was observed within the tumour lesion and the surrounding tissue, nevertheless nuclear and cytoplasmic β-catenin expression was observed in the adenomas' area and occasionally in the histologically normal adjacent tissue. Similarly heterogeneous NT1 receptor labelling was also observed, with only selected tissue areas strongly expressing the NT1 receptor.
We searched for matches between NT1 receptor expression and β-catenin cytoplasmic or nuclear expression in the same area of the adjacent tissue section and found, as summarized in Table I , a perfect correlation between nuclear or cytosolic β-catenin expression and NT1 receptor labelling in colorectal precursors lesions (adenomas and closed histologically normal adjacent mucosa) in tissues from patient with FAP, LOH or HNPCC syndromes for which it is known that APC mutation and Wnt signalling defects occur ( 33 ). When β-catenin was localized as a faint staining at the plasma membrane level, NT1 receptor detection was negative. In contrast, in patients with MSI syndrome, β-catenin was located at the cell membrane in the entire tissue section and NT1 receptor was not expressed.
Correlation between NT1 receptor expression and β-catenin localization in colonic adenomas and histologically normal adjacent mucosa
| . | FAP . | HNPCC . | LOH . | MSI . |
|---|---|---|---|---|
| . | n = 8 . | n = 8 . | n = 10 . | n = 4 . |
| NT-1 receptor (+)/−β-cat N or C | 6 | 5 | 5 | |
| NT-1 receptor (−)/−β-cat N or C | 1 | |||
| NT-1 receptor (−)/−β-cat PM | 2 | 3 | 4 | 4 |
| . | FAP . | HNPCC . | LOH . | MSI . |
|---|---|---|---|---|
| . | n = 8 . | n = 8 . | n = 10 . | n = 4 . |
| NT-1 receptor (+)/−β-cat N or C | 6 | 5 | 5 | |
| NT-1 receptor (−)/−β-cat N or C | 1 | |||
| NT-1 receptor (−)/−β-cat PM | 2 | 3 | 4 | 4 |
PM, plasma membrane; N, nuclear; C, cytosolic. For each specimen five areas highly expressing NT1 receptor were screened for β-catenin localization in the same area from adjacent tissue sections labelled for β-catenin. Within the same sample, the β-catenin localization was also evaluated from five areas not expressing NT1 receptor.
Correlation between NT1 receptor expression and β-catenin localization in colonic adenomas and histologically normal adjacent mucosa
| . | FAP . | HNPCC . | LOH . | MSI . |
|---|---|---|---|---|
| . | n = 8 . | n = 8 . | n = 10 . | n = 4 . |
| NT-1 receptor (+)/−β-cat N or C | 6 | 5 | 5 | |
| NT-1 receptor (−)/−β-cat N or C | 1 | |||
| NT-1 receptor (−)/−β-cat PM | 2 | 3 | 4 | 4 |
| . | FAP . | HNPCC . | LOH . | MSI . |
|---|---|---|---|---|
| . | n = 8 . | n = 8 . | n = 10 . | n = 4 . |
| NT-1 receptor (+)/−β-cat N or C | 6 | 5 | 5 | |
| NT-1 receptor (−)/−β-cat N or C | 1 | |||
| NT-1 receptor (−)/−β-cat PM | 2 | 3 | 4 | 4 |
PM, plasma membrane; N, nuclear; C, cytosolic. For each specimen five areas highly expressing NT1 receptor were screened for β-catenin localization in the same area from adjacent tissue sections labelled for β-catenin. Within the same sample, the β-catenin localization was also evaluated from five areas not expressing NT1 receptor.
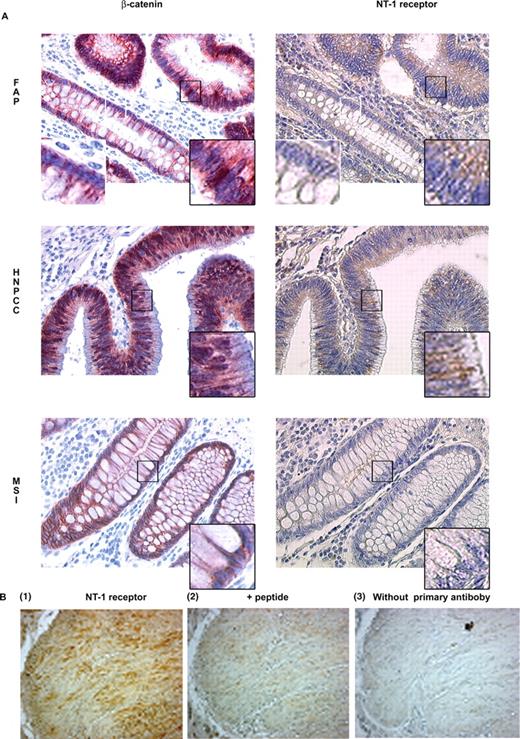
Figure 5 depicts examples of these observations. In FAP, when β-catenin was located at the plasma membrane level, NT1 receptor expression was not detected ( Figure 5A , top left and right, white inset), whereas, when β-catenin was clearly overexpressed and exhibited a cytosolic or nuclear localization, NT-1 receptor was detected as a cytoplasmic staining ( Figure 5A , top left and right, black inset). Identical observations were made in tissues from HNPCC patients ( Figure 5A , middle), and patients with sporadic LOH cancers (data not shown). The expression of NT1 receptor matched the cytoplasmic and/or nuclear accumulation of β-catenin. In patients with MSI cancers, β-catenin was located at cell–cell contacts and NT1 receptor could not be detected ( Figure 5A , bottom left and right). As a positive control, we checked for NT1 receptor expression in smooth muscle of normal human colon ( Figure 5B ). Specificity of the labelling was confirmed by displacement with the corresponding peptide. These results establish a direct correlation between NT1 receptor expression and β-catenin localization in the early stages of colon carcinogenesis.
Detection by immunohistochemistry of β-catenin and NT1 receptor in colonic adenomas. ( A ) Representative cellular distribution of β-catenin (left) and NT1 receptor (right) is shown in adenomas from FAP, HNPCC or MSI patients. The original magnification was ×400. Insets show 8-fold computerized enlargements of a region of interest, the black squares localise the images with a black contour, and the white squares localized the images with the white contour. ( B ) NT1 receptor expression in smooth muscle of normal human colon as (1) positive control, (2) preincubated with peptide or (3) without primary antibody.
Discussion
Mutations in β-catenin or APC, and critical alterations in other intermediates of the Wnt pathway, are detected at early premalignant stages during the progression of human colorectal cancers and lead to increased levels of cytoplasmic and nuclear β-catenin and subsequent activation of target genes ( 34 ). In the study, we showed that NT1 receptor expression was enhanced by Wnt agonists, the GSK-3β inhibitor lithium and forced expression of the Tcf/β-catenin complex. In its entirety, the data support the premise that β-catenin re-localization and its association with Tcf factors cause the activation of the NT1 receptor gene through the functional Tcf consensus binding site identified in its promoter. This hypothesis was verified by demonstrating that the restoration of the normal β-catenin degradation pathway by wt-APC in APC-deficient cells resulted in the impairment of the NT1 receptor expression signalling and function. Moreover the strong correlation found in the NT1 receptor expression and the β-catenin abnormal localization suggests that the activation of Wnt/APC pathway frequently observed in sporadic and familial colorectal cancers is a major cellular event leading to NT1 receptor overexpression at early stages of cell transformation.
A number of human colonic cancers are directly promoted by a β-catenin localization defect. For example, FAP syndrome is identified by its inherited APC gene mutation ( 16 , 17 ). A high mutation frequency of Tcf4 and β-catenin was found in tumours from HNPCC patients ( 33 ). The impact of APC/Wnt signalling defect in each case of colon cancer can only be estimated by abnormal cytoplasmic and nuclear β-catenin localization. In these cases we observed an excellent correlation between β-catenin localization and NT1 receptor expression in adenomas and ANM from the same tumour area ( Figure 5 and Table 1 ). The concomitant NT1 receptor expression and β-catenin nuclear and cytoplasmic localization in the ANM suggest that NT1 receptor activation is a very early event during epithelial cell transformation, as it was shown previously that cytoplasmic β-catenin accumulates in aberrant crypt foci ( 35 ).
As a consequence of NT1 receptor activation in early stages of colon tumorigenesis, we invoke the potential for NT to promote colon cancer growth and spreading. In addition to data demonstrating a proliferative role of NT in various cancers such as colon, prostate, pancreatic and lung cancer ( 12 ), it was shown that NT induces DNA synthesis in the human pancreatic carcinoma cell line, PANC-1, through the PK C-dependent PK D activation ( 36 ), as well as causing a synergistic stimulation of DNA synthesis with EGF ( 37 ). Further support is provided by the ability of NT to act as a survival factor in breast adenocarcinoma because of its ability to induce Bcl 2 protein expression ( 38 ). Besides these malignant functions, we demonstrated the ability of NT to promote HT-29 and SW480 cellular invasion in collagen type I. When these same cells are restored with wt-APC protein, NT no longer was capable of inducting invasion confirming the participation of NT1 receptor in this effect. An interpretation substantiated by the ability of a NT1 receptor specific antagonist to abrogate the invasive phenotype. Our results are in agreement with the activity of several signalling elements activated by NT via NT1 receptor and implicated in cell adhesion, activation of the cytoskeleton, and invasiveness, including Rho, focal adhesion kinase (FAK), nuclear transcription factor κB (NFκB) and mitogen activated protein kinase (MAPK) ( 7 , 8 ).
The activation of NT1 receptor in early stages of colonic tumours by its own ligand remains unknown. NT release could be locally enhanced by environmental factors, such as those induced by a high-fat diet which increases circulating NT levels for 10 h after a meal ( 10 , 39 ). Equally feasible is a paracrine or autocrine regulation occurring in the colon during the tumorigenic process, as the NT gene promoter is sensitive to Ras and Src activation ( 40 , 41 ).
A link between chronic inflammation process and colorectal cancer progression has been established since two non-steroidal anti-inflammatory drugs have been found to inhibit the growth of adenomatous polyps and cause regression of existing polyps in randomized trials of FAP patients ( 42 , 43 ). NT is also known to be a pro-inflammatory neuropeptide in colonic inflammation. It has been shown that NT and its receptor are elevated in the rat colonic mucosa following toxin A administration. Pretreatment of rats with the NT1 receptor antagonist SR 48 692 inhibits toxin A-induced changes in colonic secretion, mucosal permeability and histologic damage. These effects are mediated by NT-induced release of substance P ( 44 ). NT is also known to mediate interleukin (IL)-8 and transforming growth factor (TGF)-α release in cell culture by mechanisms implicating NFκB and matrix metalloproteinase (MMP), respectively ( 7 , 45 , 46 ), and cyclooxygenase (Cox)-2 activation in chronic intestinal inflammation ( 47 ).
Invoking the classical model of multistep colorectal carcinogenesis, we postulate that early alterations in Wnt/APC pathway lead to abnormal β-catenin localization, and subsequently to NT1 receptor expression in colonic epithelial cells. Later events controlled by other genetic alterations combined with nutritional factors could lead to enhanced expression and release of NT.
The authors thank Dr D. Caput and Dr D. Gully (Sanofi-aventis) for providing the NT1 receptor promoter clone and the NT1 receptor antagonist, SR 48692, respectively, Dr H. Clevers for providing β-catenin and Tcf-4 and Dr J. Kitajewski for providing HA-Wnt-2 expression vector, Dr Vogelstein for providing HT-29 APC and HT-29 β-gal cells. The authors greatly appreciate the help of Dr A Nicot for his expertise in performing the calcium measurements. We wish to thank Sylvie Dumont for her help in developing our slides. The authors wish to express many thanks to Dr Neil Insdorf for his help in the writing of the manuscript and for helpful discussions. This work has been supported by INSERM, ‘Association pour la Recherche sur le Cancer’ Grant (no. 3543) and ‘Ligue nationale contre le cancer’ Grant (75-00-RS40), France, and Fortis Bank Verzekeringen, Brussels. Mireille Toy-Miou-Leong was supported by INSERM and ‘ARC’.
Conflict of Interest Statement : None declared.
References
Heasley,L.E. (
Vogt,S., Grosse,R., Schultz,G. and Offermanns,S. (
Jaffe,A.B. and Hall,A. (
Lozano,E., Betson,M. and Braga,V.M. (
Nguyen,Q.D., Faivre,S., Bruyneel,E., Rivat,C., Seto,M., Endo,T., Mareel,M., Emami,S. and Gespach,C. (
Najimi,M., Maloteaux,J.M. and Hermans,E. (
Zhao,D., Kuhnt-Moore,S., Zeng,H., Wu,J.S., Moyer,M.P. and Pothoulakis,C. (
Leyton,J., Garcia-Marin,L., Jensen,R.T. and Moody,T.W. (
Reinecke,M. (
Draviam,E.J., Upp,J.R., Jr., Greeley,G.H., Jr., Townsend,C.M., Jr. and Thompson,J.C. (
Giovannucci,E. (
Thomas,R.P., Hellmich,M.R., Townsend,C.M., Jr. and Evers,B.M. (
Maoret,J.J., Anini,Y., Rouyer-Fessard,C., Gully,D. and Laburthe,M. (
Moody,T.W., Chiles,J., Casibang,M., Moody,E., Chan,D. and Davis,T.P. (
Maoret,J.J., Pospai,D., Rouyer-Fessard,C., Couvineau,A., Laboisse,C., Voisin,T. and Laburthe,M. (
Kinzler,K.W. and Vogelstein,B. (
Morin,P.J., Sparks,A.B., Korinek,V., Barker,N., Clevers,H., Vogelstein,B. and Kinzler,K.W. (
van Es,J.H., Barker,N. and Clevers,H. (
Faux,M.C., Ross,J.L., Meeker,C., Johns,T., Ji,H., Simpson,R.J., Layton,M.J. and Burgess,A.W. (
Gompel,A., Malet,C., Spritzer,P., Lalardrie,J.P., Kuttenn,F. and Mauvais-Jarvis,P. (
Sambrook,J., Fritsch,E.F. and Maniatis,T. (
Korinek,V., Barker,N., Morin,P.J., van Wichen,D., de Weger,R., Kinzler,K.W., Vogelstein,B. and Clevers,H. (
Therrien,M. and Drouin,J. (
Souaze,F., Rostene,W. and Forgez,P. (
Su,L.K., Vogelstein,B. and Kinzler,K.W. (
Boudin,H., Grauz-Guyon,A., Faure,M.P., Forgez,P., Lhiaubet,A.M., Dennis,M., Beaudet,A., Rostene,W. and Pelaprat,D. (
Kurnellas,M.P., Nicot,A., Shull,G.E. and Elkabes,S. (
Bracke,M.E, Boterberg,T., Bruyneel E.A. and Mareel M.M. (
Jourdan,F., Sebbagh,N., Comperat,E., Mourra,N., Flahault,A., Olschwang,S., Duval,A., Hamelin,R. and Flejou,J.F. (
Cagatay,T. and Ozturk,M. (
Le,F., Groshan,K., Zeng,X.P. and Richelson,E. (
Brabletz,T., Jung,A., Hermann,K., Gunther,K., Hohenberger,W. and Kirchner,T. (
Miyaki,M., Iijima,T., Kimura,J., Yasuno,M., Mori,T., Hayashi,Y., Koike,M., Shitara,N., Iwama,T. and Kuroki,T. (
Fodde,R., Smits,R. and Clevers,H. (
van de Wetering,M., Sancho,E., Verweij,C. et al . (
Guha,S., Rey,O. and Rozengurt,E. (
Kisfalvi,K., Guha,S. and Rozengurt,E. (
Somai,S., Gompel,A., Rostene,W. and Forgez,P. (
Gullo,L., Pezzilli,R., Tomassetti,P. and de Giorgio,R.P. (
Banker,N.A., Hellmich,M.R., Kim,H.J., Townsend,C.M., Jr. and Evers,B.M. (
Evers,B.M., Zhou,Z., Celano,P. and Li,J. (
Giardiello,F.M., Hamilton,S.R., Krush,A.J., Piantadosi,S., Hylind,L.M., Celano,P., Booker,S.V., Robinson,C.R. and Offerhaus,G.J. (
Steinbach,G., Lynch,P.M., Phillips,R.K. et al . (
Castagliuolo,I., Wang,C.C., Valenick,L., Pasha,A., Nikulasson,S., Carraway,R.E. and Pothoulakis,C. (
Zhao,D., Zhan,Y., Zeng,H., Koon,H.W., Moyer,M.P. and Pothoulakis,C. (
Zhao,D., Zhan,Y., Koon,H.W., Zeng,H., Keates,S., Moyer,M.P. and Pothoulakis,C. (
Author notes
INSERM U673–UPMC, 1Department of Pathology, and 2INSERM U732–UPMC, Hôpital Saint-Antoine, 184 Rue Du Faubourg Saint-Antoine, 75571 Paris Cedex 12, France, 3The Laboratory of Experimental Cancerology, Ghent University Hospital, B-9000 Ghent, Belgium and 4Ludwig Institute for Cancer Research, Parkville, Victoria 3050, Australia
- phenotype
- transcription, genetic
- calcium
- colorectal cancer
- familial adenomatous polyposis
- adenoma
- cell lines
- hereditary nonpolyposis colorectal neoplasms
- cytoplasm
- cytosol
- genes
- ht29 cells
- intercellular junctions
- loss of heterozygosity
- neuropeptides
- neurotensin
- receptors, neurotensin
- response elements
- t-lymphocytes
- up-regulation (physiology)
- colon
- neoplasms
- colon cancer
- g-protein-coupled receptors
- gastrointestinal tract
- carcinogenesis
- epithelial cells
- signal pathway
- signal transduction pathways
- gene activation
- tumor cells, malignant
- adenomatous polyp of colon


![NT1 receptor is a Tcf-4/β-catenin target gene. ( A ) Luciferase reporter activity of NT1 receptor promoter. Cos-7 cells were transiently transfected with wild-type (wt), or mutated promoter-luciferase reporter constructs, or empty vector control plasmid, together with β-catenin and/or hTcf4 expression vectors. The results were expressed as fold induction, and in each measurement luciferase activity was divided by β-galactosidase activity and protein content. Results are the mean of four independent experiments. ( B ) Electrophoretic mobility shift assay of mock (lanes 1, 3 and 5) or Tcf/β-catenin (lanes 2, 4 and 6–9) transfected Cos-7 nuclear extracts, with [γ- 32 P]ATP-labelled probes. Lanes 1–8, wild-type probe (Wt Tcf); lane 9, mutated probe in the putative Tcf-4 DNA binding site (Mut Tcf). In lanes 3 and 4, the Tcf-4 antibody was added to the nuclear lysates. Lanes 7 and 8 show competition with a 100-fold excess of wild-type and mutated cold probe, respectively. This is a representative experiment of three independent experiments. ( C ) Accumulation of endogenous NT1 receptor transcripts detected by RT–PCR following over-expression β-catenin in HEK 293T cells, or treatment of normal HBEC with Wnt factors or LiCl. HBEC were incubated for 48 h with cellular extract from mock or Wnt-2 or Wnt-7b-transfected HEK 293T cells. This is a representative experiment of three independent experiments. ( D ) β-catenin immunocytochemistry (red) and nuclear DAPI (blue) staining of HBEC control, or treated, for 6 h, with cellular extract from pcDNA3 or Wnt-7b-transfected HEK-293T cells, or 20 mM LiCl.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/carcin/27/4/10.1093_carcin_bgi269/2/m_bgi269f1.jpeg?Expires=1716549814&Signature=FdcCjchvFbDEvUeJ6S5fcO5-btcr14R5P-sxQMBbk90EEr4uw5UxQt6OUJcYzGjvr9u0w4ouW8eMhtXB3nAvtwvWikreIaopW~GUZ9ALszk2vWpT-AC5CcH8YO6AIgPvzqIBNnuK4C2Kuyvbr64dFAlu8~kd5oF7ForUlNWnKCHSsSBYF47r-fcOrB-V6sdsDEZaQ7RxgPpbrIu9HFAPFKQQBqO7e4t8gzY6HSvrTEXgfLj0VT71fFpyJFla6CEqsFvw30P74yZBmt5gJTbDdXGVYro8Tg6SJwSveBHpQ9zdHJO3QSlAaUPZTEOBQj103RxsIasM~i90fHv5qLyIYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)