Abstract
Rationale
Recent studies provide evidence for reduced phosphodiesterase-4B (PDE4B) as a genetic susceptibility factor as well as suggesting an association of several single nucleotide polymorphisms (SNPs) in PDE4B that are associated with an increased incidence of schizophrenia.
Objectives
The aim of the current study was to assess the activity of rolipram, a nonsubtype-selective PDE4 inhibitor, in several animal models predictive of antipsychotic-like efficacy and side-effect liability and to use PDE4B wild-type and knockout mice to begin to understand the subtypes involved in the activity of rolipram.
Results
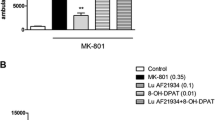
In rats, rolipram antagonized both phencyclidine hydrochloride- and d-amphetamine-induced hyperactivity and inhibited conditioned avoidance responding (CAR). In PDE4B wild-type mice, rolipram dose-dependently suppressed CAR (ED50 = 2.4 mg/kg); however, in knockout mice, their sensitivity to rolipram at the higher doses (1.0 and 3.2 mg/kg) was reduced, resulting in a threefold shift in the ED50 (7.3 mg/kg), suggesting PDE4B is involved, at least in part, with the activity of rolipram. Only the highest dose of rolipram (3.2 mg/kg) produced a modest but significant degree of catalepsy.
Conclusions
Rolipram has a pharmacologic profile similar to that of the atypical antipsychotics and has low extrapyramidal symptom liability. These results suggest that PDE4B mediates the antipsychotic effects of rolipram in CAR and that the PDE4B-regulated cyclic adenosine monophosphate signaling pathway may play a role in the pathophysiology and pharmacotherapy of psychosis.





Similar content being viewed by others
References
Abi-Saab WM, D’Souza DC, Moghaddam B, Krystal JH (1998) The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry 31:104–109
Beavo JA (1995) Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Res 75(4):725–748
Blokland A, Schreiber R, Prickaerts J (2006) Improving memory: a role for phosphodiesterases. Curr Pharm Des 12(20):2511–2523
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D (1997) Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 94:2569–2574
Carlsson A (1988) The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1:179–186
Carlsson A (2001) A half-century of neurotransmitter research: impact on neurology and psychiatry. Nobel lecture. Biosci Rep 21:691–710
Carlsson ML, Carlsson A, Nilsson M (2004) Schizophrenia: from dopamine to glutamate and back. Curr Med Chem 11:267–277
Cherry JA, Davis RL (1999) Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol 407:287–301
Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C (2003) Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem 278:5493–5496
Costall B, Fortune DH, Naylor RJ, Mardsen CD, Pycock C (1975) Serotonergic involvement with neuroleptic catalepsy. Neuropharmacology 14:859–868
Crocker AD, Russell RW (1984) The up-and-down method for the determination of nociceptive thresholds in rats. Pharmacol Biochem Behav 21:133–136
Davis JA, Gould TJ (2005) Rolipram attenuates MK-801-induced deficits in latent inhibition. Behav Neurosci 119:595–602
Ghavami A, Hirst WD, Novak TJ (2006) Selective phosphodiesterase (PDE)-4 inhibitors: a novel approach to treating memory deficit? Drugs R&D 7:63–71
Goff DC, Coyle JT (2001) The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 158:1367–1377
Graybiel AM (2000) The basal ganglia. Curr Biol 10:R509–R511
Halberstadt AL (1995) The phencyclidine–glutamate model of schizophrenia. Clin Neuropharmacol 18:237–249
Houslay MD (2001) PDE4 cAMP-specific phosphodiesterases. Prog Nucleic Acid Res Mol Biol 69:249–315
Houslay MD, Schafer P, Zhang KY (2005) Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today 10:1503–1519
Iona S, Cuomo M, Bushnik T, Naro F, Sette C, Hess M, Shelton ER, Conti M (1998) Characterization of the rolipram-sensitive, cyclic AMP-specific phosphodiesterases: identification and differential expression of immunologically distinct forms in the rat brain. Mol Pharmacol 53:23–32
Iyo M, Bi Y, Hashimoto K, Inada T, Fukui S (1996) Prevention of methamphetamine-induced behavioral sensitization in rats by a cyclic AMP phosphodiesterase inhibitor, rolipram. Eur J Pharmacol 312:163–170
Jentsch JD, Roth RH (1999) The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20:201–225
Jin S-LC, Richard FJ, Kuo WP, D’Ercole AJ, Conti M (1999) Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci USA 96:11998–12003
Jin SL, Latour AM, Conti M (2005) Generation of PDE4 knockout mice by gene targeting. Methods Mol Biol 307:191–210
Kaneko M, Sato K, Horikoshi R, Yaginuma M, Yagimuma N, Shiragata M, Kumashiro H (1992) Effects of haloperidol on cyclic AMP and inositol trisphosphate in rat striatum in vivo. Prostaglandins Leukot Essent Fat Acids 46:53–57
Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP (2006) Rolipram: a specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience 144:239–246 (Epub 2006 Nov 1)
Kehr W, Debus G, Neumeister R (1985) Effects of rolipram, a novel antidepressant, on monoamine metabolism in rat brain. J Neural Transm 63:1–12
King DP, Paciga SA, Fan Y, Menniti FS (2006) Positive genetic association of phosphodiesterase 4B (PDE4B) with schizophrenia: analysis in two case-control populations. Program no. 94.20, Neuroscience Meeting Planner. Society for Neuroscience, Atlanta, GA (Abstracts Online)
Krause W, Kuhne G (1988) Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica 18:561–571
Krause W, Kuhne G (1993) Biotransformation of the antidepressant D,L-rolipram. II. Metabolite patterns in man, rat, rabbit, rhesus and cynomolgus monkey. Xenobiotica 23:1277–1288
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, DeSouza CD, Endos I (1996) Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 93:9235–9240
Lobban M, Shakur Y, Beattie J, Houslay MD (1994) Identification of two splice variant forms of type-IVB cyclic AMP phosphodiesterase, DPD (rPDE-IVB1) and PDE-4 (rPDE-IVB2) in brain: selective localization in membrane and cytosolic compartments and differential expression in various brain regions. Biochem J 304:399–406
MacKenzie SJ, Houslay M (2000) Action of rolipram on specific PDE4 cAMP phosphodiesterase isoforms and on the phosphorylation of cAMP-response-element-binding protein (CREB) and p38 mitogen-activated protein (MAP) kinase in U937 monocytic cells. Biochem J 347:571–578
Maxwell CR, Kanes SJ, Abel T, Siegel SJ (2004) Phosphodiesterase inhibitors: a novel mechanism for receptor-independent antipsychotic medications. Neuroscience 129:101–107
McPhee I, Pooley L, Lobban M, Bolger G, Houslay MD (1995) Identification, characterization and regional distribution in brain of RPDE-6 (RNPDE4A5), a novel splice variant of the PDE4A cyclic AMP phosphodiesterase family. Biochem J 310:965–974
Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, Rain JC, Camargo LM, Whiting PJ, Houslay MD, Blackwood DH, Muir WJ, Porteous DJ (2005) DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 31:1187–1191
Mori T, Baba J, Ichimaru Y, Suzuki T (2000) Effects of rolipram, a selective inhibitor of phosphodiesterase 4, on hyperlocomotion induced by several abused drugs in mice. Jpn J Pharmacol 83:113–118
Morris BJ, Cochran SM, Pratt JA (2005) PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol 5:101–106
National Research Council (1996) Guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources. National Academy Press, Washington, DC
Olney JW, Farber NB (1995) Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52:998–1007
Onali P, Olianas MC, Gessa GL (1985) Characterization of dopamine receptors mediating inhibition of adenylate cyclase activity in rat striatum. Mol Pharmacol 28:138–145
Pearlson GD (2000) Neurobiology of schizophrenia. Ann Neurol 48:556–566
Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G (2000) Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat 20:349–374
Pietzcker A, Muller-Oerlinghausen B, Kehr W (1979) Antipsychotic activity of rolipram in schizophrenic patients. A pilot study. Naunyn-Schmiedebergs Arch Pharmacol 308:R44
Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, Liu S, Huang Z, Conti M, Chan CC (2002) Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest 110:1045–1152
Schneider HH (1984) Brain cAMP response to phosphodiesterase inhibitors in rats killed by microwave irradiation or decapitation. Biochem Pharmacol 33:1690–1693
Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL et al (2003) Immunohistochemical localization of PDE10A in the rat brain. Brain Res 985:113–126
Siuciak JA, McCarthy SA, Chapin DS, Fujiwara RA, James LC, Williams RD, Stock JL, McNeish JD, Strick CA, Menniti FS, Schmidt CJ (2006a) Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: evidence for altered striatal function. Neuropharmacology 51:374–385
Siuciak JA, Chapin DS, Harms JF, Lebel LA, McCarthy SA, Chambers L et al (2006b) Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology 51:386–396
Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P et al (2007) CP-809,101, a selective 5-HT(2C) agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 52(2):279–290 (available online 1 Sep 2006)
Soderling SH, Bayuga SJ, Beavo JA (1999) Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc Natl Acad Sci USA 96:7071–7076
Tamminga CA (1998) Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 12:21–36
Wachtel H (1983) Potential antidepressant activity of rolipram and other selective cyclic adenosine 3′,5′-monophosphate phosphodiesterase inhibitors. Neuropharmacology 22:267–272
Wadenberg ML, Hicks PB (1999) The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev 23:851–862
Wang SJ (2006) An investigation into the effect of the type IV phosphodiesterase inhibitor rolipram in the modulation of glutamate release from rat prefrontocortical nerve terminals. Synapse 59:41–50
Wong AH, Van Tol HH (2003) Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev 27:269–306
Zhang KY, Ibrahim PN, Gillette S, Bollag G (2005) Phosphodiesterase-4 as a potential drug target. Expert Opin Ther Targets 9:1283–1305
Acknowledgments
The authors would like to thank Dr. Marco Conti for supplying the breeding pairs of PDE4B WT and KO mice used to initiate our colonies and Linda Loverro and the Genetically Modified Mice Group for their assistance in the breeding, colony expansion, and delivery of the knockout mice. Portions of this work were previously presented at the Society for Neuroscience Meeting, Atlanta, GA, October, 2006, and the International Congress on Schizophrenia Research, Colorado Springs, CO, April, 2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siuciak, J.A., Chapin, D.S., McCarthy, S.A. et al. Antipsychotic profile of rolipram: efficacy in rats and reduced sensitivity in mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology 192, 415–424 (2007). https://doi.org/10.1007/s00213-007-0727-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0727-x