Abstract
Object
To examine the whole brain white matter morphology in antipsychotic-naive patients with first-episode schizophrenia (FES) and its correlations with symptom severity.
Materials and methods
High-resolution T1-weighted images of 64 drug-naive FES patients and 64 matched healthy controls were acquired using a 3 T MR imaging system. Then, optimized voxel-based morphometry was performed to compare the group differences. Finally, correlation analyses were conducted between the white matter volume (WMV) changes and clinical symptoms.
Results
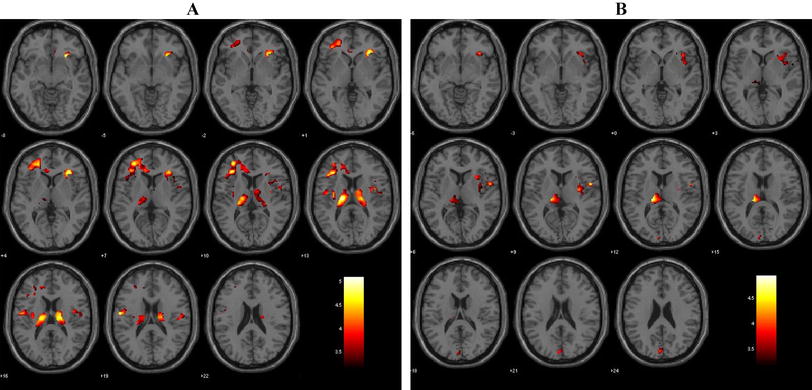
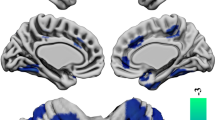
The FES showed significantly decreased WMV in the bilateral posterior limb of the internal capsule (PLIC) and right subgyral frontal white matter. The volume of the bilateral PLIC was negatively correlated with the Positive and Negative Syndrome Scale positive scores. Positive correlations were observed between all of the changed WMV measures and the Global Assessment of Functioning scores.
Conclusion
The current findings provide further evidence to support internal capsule and subgyral frontal white matter deficits at the early stage of schizophrenia that are potentially related to the core pathophysiology of the disease. Furthermore, these anatomical alterations were related to the clinical symptoms but not the untreated illness duration, suggesting that these deficits are related to aberrations in the neurodevelopmental process and may be relatively stable during the early course of schizophrenia.

Similar content being viewed by others
References
Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, Zhang T, Li X, Li D, Zou L, Tang H, Zhou XJ, Mechelli A, Collier DA, Sweeney JA, Li T, Gong Q (2009) Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry 166(2):196–205
Honea R, Crow TJ, Passingham D, Mackay CE (2005) Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 162(12):2233–2245
Kim JJ, Crespo-Facorro B, Andreasen NC, O’Leary DS, Magnotta V, Nopoulos P (2003) Morphology of the lateral superior temporal gyrus in neuroleptic naive patients with schizophrenia: relationship to symptoms. Schizophr Res 60(2–3):173–181
Whitford TJ, Farrow TF, Gomes L, Brennan J, Harris AW, Williams LM (2005) Grey matter deficits and symptom profile in first episode schizophrenia. Psychiatry Res 139(3):229–238
Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC (2000) Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry 57(8):761–768
Spalletta G, Tomaiuolo F, Marino V, Bonaviri G, Trequattrini A, Caltagirone C (2003) Chronic schizophrenia as a brain misconnection syndrome: a white matter voxel-based morphometry study. Schizophr Res 64(1):15–23
Whitford TJ, Grieve SM, Farrow TF, Gomes L, Brennan J, Harris AW, Gordon E, Williams LM (2007) Volumetric white matter abnormalities in first-episode schizophrenia: a longitudinal, tensor-based morphometry study. Am J Psychiatry 164(7):1082–1089
Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO (2005) White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry 162(3):602–605
Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V (2003) White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry 60(5):443–456
McDonald C, Bullmore E, Sham P, Chitnis X, Suckling J, MacCabe J, Walshe M, Murray RM (2005) Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry 186:369–377
Buchanan RW, Vladar K, Barta PE, Pearlson GD (1998) Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry 155(8):1049–1055
Witthaus H, Brune M, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, Heinz A, Klingebiel R, Juckel G (2008) White matter abnormalities in subjects at ultra high-risk for schizophrenia and first-episode schizophrenic patients. Schizophr Res 102(1–3):141–149
Hulshoff Pol HE, Schnack HG, Mandl RCW, Cahn W, Collins DL, Evans AC, Kahn RS (2004) Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. NeuroImage 21(1):27–35
Suzuki M, Nohara S, Hagino H, Kurokawa K, Yotsutsuji T, Kawasaki Y, Takahashi T, Matsui M, Watanabe N, Seto H, Kurachi M (2002) Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr Res 55(1–2):41–54
Frumin M, Golland P, Kikinis R, Hirayasu Y, Salisbury DF, Hennen J, Dickey CC, Anderson M, Jolesz FA, Grimson WE, McCarley RW, Shenton ME (2002) Shape differences in the corpus callosum in first-episode schizophrenia and first-episode psychotic affective disorder. Am J Psychiatry 159(5):866–868
Bagary MS, Symms MR, Barker GJ, Mutsatsa SH, Joyce EM, Ron MA (2003) Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry 60(8):779–788
Thomann PA, Wustenberg T, Santos VD, Bachmann S, Essig M, Schroder J (2009) Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol Med 39(3):371–379
Lee KH, Farrow TF, Parks RW, Newton LD, Mir NU, Egleston PN, Brown WH, Wilkinson ID, Woodruff PW (2007) Increased cerebellar vermis white-matter volume in men with schizophrenia. J Psychiatr Res 41(8):645–651
Salokangas RK, Cannon T, Van Erp T, Ilonen T, Taiminen T, Karlsson H, Lauerma H, Leinonen KM, Wallenius E, Kaljonen A, Syvalahti E, Vilkman H, Alanen A, Hietala J (2002) Structural magnetic resonance imaging in patients with first-episode schizophrenia, psychotic and severe non-psychotic depression and healthy controls. Results of the schizophrenia and affective psychoses (SAP) project. Br J Psychiatry Suppl 43:s58–s65
Filley CM (2005) White matter and behavioral neurology. Ann N Y Acad Sci 1064:162–183
Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M (1999) Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 46(7):908–920
McGuire PK, Frith CD (1996) Disordered functional connectivity in schizophrenia. Psychol Med 26(4):663–667
Ashburner J, Friston KJ (2000) Voxel-based morphometry: the methods. NeuroImage 11(6 Pt 1):805–821
Chua SE, Cheung C, Cheung V, Tsang JT, Chen EY, Wong JC, Cheung JP, Yip L, Tai KS, Suckling J, McAlonan GM (2007) Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res 89(1–3):12–21
Ashburner J, Friston KJ (2001) Why voxel-based morphometry should be used. NeuroImage 14(6):1238–1243
Giuliani NR, Calhoun VD, Pearlson GD, Francis A, Buchanan RW (2005) Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr Res 74(2–3):135–147
Annett M (1970) A classification of hand preference by association analysis. Br J Psychol 61(3):303–321
Goldman HH, Skodol AE, Lave TR (1992) Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry 149(9):1148–1156
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13(2):261–276
Kay SR, Opler LA, Lindenmayer JP (1988) Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res 23(1):99–110
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14(1 Pt 1):21–36
Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S (2003) Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 362(9386):798–805
Georgieva L, Moskvina V, Peirce T, Norton N, Bray NJ, Jones L, Holmans P, Macgregor S, Zammit S, Wilkinson J, Williams H, Nikolov I, Williams N, Ivanov D, Davis KL, Haroutunian V, Buxbaum JD, Craddock N, Kirov G, Owen MJ, O’Donovan MC (2006) Convergent evidence that oligodendrocyte lineage transcription factor 2 (OLIG2) and interacting genes influence susceptibility to schizophrenia. Proc Natl Acad Sci USA 103(33):12469–12474
Wobrock T, Gruber O, Schneider-Axmann T, Wolwer W, Gaebel W, Riesbeck M, Maier W, Klosterkotter J, Schneider F, Buchkremer G, Moller HJ, Schmitt A, Bender S, Schlosser R, Falkai P (2009) Internal capsule size associated with outcome in first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci 259(5):278–283
Filippi M, Canu E, Gasparotti R, Agosta F, Valsecchi P, Lodoli G, Galluzzo A, Comi G, Sacchetti E (2013) Patterns of brain structural changes in first-contact, antipsychotic drug-naive patients with schizophrenia. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A3583
Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T, Isoda H, Tsuchiya KJ, Takebayashi K, Suzuki K, Sakahara H, Nakamura K, Mori N, Takei N (2008) Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Ann Gen Psychiatry 7:25
Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE (2008) A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med 38(6):877–885
Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS (2007) Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res 92(1–3):211–224
Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yucel M, Velakoulis D, Pantelis C (2011) Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 127(1–3):46–57
Axer H, Keyserlingk DG (2000) Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. J Neurosci Methods 94(2):165–175
Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME (2005) DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. NeuroImage 26(4):1109–1118
Zhang T, Davatzikos C (2013) Optimally-discriminative voxel-based morphometry significantly increases the ability to detect group differences in schizophrenia, mild cognitive impairment, and Alzheimer’s disease. NeuroImage 79:94–110
Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Matsui M, Seto H, Kurachi M (2005) Volumetric analysis of sulci/gyri-defined in vivo frontal lobe regions in schizophrenia: precentral gyrus, cingulate gyrus, and prefrontal region. Psychiatry Res 139(2):127–139
Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q (2010) Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry 67(8):783–792
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101(21):8174–8179
Casey BJ, Jones RM, Hare TA (2008) The adolescent brain. Ann N Y Acad Sci 1124:111–126
Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC (2011) Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 70(7):672–679
de Weijer AD, Mandl RC, Diederen KM, Neggers SF, Kahn RS, Hulshoff Pol HE, Sommer IE (2011) Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr Res 130(1–3):68–77
Abdul-Rahman MF, Qiu A, Woon PS, Kuswanto C, Collinson SL, Sim K (2012) Arcuate fasciculus abnormalities and their relationship with psychotic symptoms in schizophrenia. PLoS One 7(1):e29315
Rotarska-Jagiela A, Oertel-Knoechel V, DeMartino F, van de Ven V, Formisano E, Roebroeck A, Rami A, Schoenmeyer R, Haenschel C, Hendler T, Maurer K, Vogeley K, Linden DE (2009) Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res 174(1):9–16
Davatzikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, Hughett P, Turetsky BI, Gur RE (2005) Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry 62(11):1218–1227
Boos HB, Mandl RC, van Haren NE, Cahn W, van Baal GC, Kahn RS, Hulshoff Pol HE (2013) Tract-based diffusion tensor imaging in patients with schizophrenia and their non-psychotic siblings. Eur Neuropsychopharmacol 23(4):295–304
Kraepelin E (1971) Dementia praecox and paraphrenia. Krieger Publishing Company, Chicago
van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Brans R, Carati I, Rais M, Kahn RS (2008) Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry 63(1):106–113
Vita A, De Peri L, Deste G, Sacchetti E (2012) Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry 2:e190
Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL (2001) Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA 98(20):11650–11655
Arango C, Rapado-Castro M, Reig S, Castro-Fornieles J, Gonzalez-Pinto A, Otero S, Baeza I, Moreno C, Graell M, Janssen J, Parellada M, Moreno D, Bargallo N, Desco M (2012) Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry 69(1):16–26
Gold S, Arndt S, Nopoulos P, O’Leary DS, Andreasen NC (1999) Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry 156(9):1342–1348
Zipursky RB, Reilly TJ, Murray RM (2012) The myth of schizophrenia as a progressive brain disease. Schizophr Bull. doi:10.1093/schbul/sbs135
Weinberger DR, McClure RK (2002) Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch Gen Psychiatry 59(6):553–558
Acknowledgments
This work was supported by the National Natural Science Foundation (Grants 81222018, 81030027, 81227002, and 81220108013); the Programs for New Century Excellent Talents in University (Grant no. NCET-10-0596); Young Scholars of Sichuan (Grant no. 2011JQ0005); the Distinguished Professorship awarded to Dr. Qiyong Gong by the China Medical Board administered by the Institute of International Education, Washington, DC; the Program for Changjiang Scholars and Innovative Research Team in University of China; and the National Key Technologies R&D Program of China (Program 2012BAI01B03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Li Yao and Min Wu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yao, L., Lui, S., Deng, W. et al. Association of white matter deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized VBM study using 3T. Magn Reson Mater Phy 27, 283–290 (2014). https://doi.org/10.1007/s10334-013-0411-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-013-0411-6