Abstract
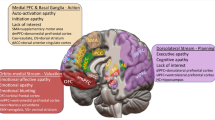
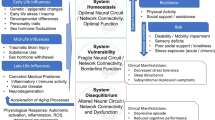
Deficits in cognition, reward processing, and motor function are clinical features relevant to both aging and depression. Individuals with late-life depression often show impairment across these domains, all of which are moderated by the functioning of dopaminergic circuits. As dopaminergic function declines with normal aging and increased inflammatory burden, the role of dopamine may be particularly salient for late-life depression. We review the literature examining the role of dopamine in the pathogenesis of depression, as well as how dopamine function changes with aging and is influenced by inflammation. Applying a Research Domain Criteria (RDoC) Initiative perspective, we then review work examining how dopaminergic signaling affects these domains, specifically focusing on Cognitive, Positive Valence, and Sensorimotor Systems. We propose a unified model incorporating the effects of aging and low-grade inflammation on dopaminergic functioning, with a resulting negative effect on cognition, reward processing, and motor function. Interplay between these systems may influence development of a depressive phenotype, with an initial deficit in one domain reinforcing decline in others. This model extends RDoC concepts into late-life depression while also providing opportunities for novel and personalized interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Taylor WD. Clinical practice. Depression in the elderly. N. Engl J Med. 2014;371:1228–36.
Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–74.
Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–31.
Hybels CF, Landerman LR, Blazer DG. Age differences in symptom expression in patients with major depression. Int J Geriatr Psychiatry. 2012;27:601–11.
Rutherford BR, Taylor WD, Brown PJ, Sneed JR, Roose SP. Biological Aging and the Future of Geriatric Psychiatry. J Gerontol A Biol Sci Med Sci. 2017;72:343–52.
Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–8.
Seaman KL, Smith CT, Juarez EJ, Dang LC, Castrellon JJ, Burgess LL, et al. Differential regional decline in dopamine receptor availability across adulthood: Linear and nonlinear effects of age. Hum Brain Mapp. 2019;40:3125–38.
Karrer TM, Josef AK, Mata R, Morris ED, Samanez-Larkin GR. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging. 2017;57:36–46.
Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27:327–38.
Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–37.
Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63.
Smith CC, Greene RW. CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci. 2012;32:6072–80.
Devoto P, Flore G. On the origin of cortical dopamine: is it a co-transmitter in noradrenergic neurons? Curr Neuropharmacol. 2006;4:115–25.
Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci USA. 2016;113:14835–40.
Yamasaki M, Takeuchi T. Locus Coeruleus and Dopamine-Dependent Memory Consolidation. Neural Plast. 2017;2017:8602690.
Nestler EJ, Carlezon WA Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9.
Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, et al. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Commun. 2016;7:11829.
Salamone JD, Pardo M, Yohn SE, Lopez-Cruz L, SanMiguel N, Correa M. Mesolimbic Dopamine and the Regulation of Motivated Behavior. Curr Top. Behav Neurosci. 2016;27:231–57.
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–41.
Pecina M, Sikora M, Avery ET, Heffernan J, Pecina S, Mickey BJ, et al. Striatal dopamine D2/3 receptor-mediated neurotransmission in major depression: Implications for anhedonia, anxiety and treatment response. Eur Neuropsychopharmacol. 2017;27:977–86.
Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NP, et al. Elevated putamen D(2) receptor binding potential in major depression with motor retardation: an [11C]raclopride positron emission tomography study. Am J Psychiatry. 2006;163:1594–602.
Hamilton JP, Sacchet MD, Hjornevik T, Chin FT, Shen B, Kampe R, et al. Striatal dopamine deficits predict reductions in striatal functional connectivity in major depression: a concurrent (11)C-raclopride positron emission tomography and functional magnetic resonance imaging investigation. Transl Psychiatry. 2018;8:264.
Parsey RV, Oquendo MA, Zea-Ponce Y, Rodenhiser J, Kegeles LS, Pratap M, et al. Dopamine D(2) receptor availability and amphetamine-induced dopamine release in unipolar depression. Biol Psychiatry. 2001;50:313–22.
Schneier FR, Slifstein M, Whitton AE, Pizzagalli DA, Reinen J, McGrath PJ, et al. Dopamine release in antidepressant-naive major depressive disorder: a multimodal [(11)C]-(+)-PHNO positron emission tomography and functional magnetic resonance imaging study. Biol Psychiatry. 2018;84:563–73.
Li Z, He Y, Tang J, Zong X, Hu M, Chen X. Molecular imaging of striatal dopamine transporters in major depression-a meta-analysis. J Affect Disord. 2015;174:137–43.
Pizzagalli DA, Berretta S, Wooten D, Goer F, Pilobello KT, Kumar P, et al. Assessment of striatal dopamine transporter binding in individuals with major depressive disorder: in vivo positron emission tomography and postmortem evidence. JAMA psychiatry. 2019;76:854–61.
Dubol M, Trichard C, Leroy C, Granger B, Tzavara ET, Martinot JL, et al. Lower midbrain dopamine transporter availability in depressed patients: report from high-resolution PET imaging. J Affect Disord. 2020;262:273–7.
Moriya H, Tiger M, Tateno A, Sakayori T, Masuoka T, Kim W, et al. Low dopamine transporter binding in the nucleus accumbens in geriatric patients with severe depression. Psychiatry Clin Neurosci. 2020;74:424–30.
Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–9.
Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–20.
van Dyck CH, Arnsten AFT, Padala PR, Brawman-Mintzer O, Lerner AJ, Porsteinsson AP, et al. Neurobiologic rationale for treatment of apathy in Alzheimer’s disease with methylphenidate. Am J Geriatr Psychiatry. 2021;29:51–62.
Yuen GS, Bhutani S, Lucas BJ, Gunning FM, AbdelMalak B, Seirup JK, et al. Apathy in late-life depression: common, persistent, and disabling. Am J Geriatr Psychiatry. 2015;23:488–94.
Krishnan KRR, Hays JC, Tupler LA, George LK, Blazer DG. Clinical and phenomenological comparisons of late-onset and early-onset depression. Am J Psychiatry. 1995;152:785–8.
Mehta M, Whyte E, Lenze E, Hardy S, Roumani Y, Subashan P, et al. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young-old and old-old. Int J Geriatr Psychiatry. 2008;23:238–43.
Stark AK, Pakkenberg B. Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res. 2004;318:81–92.
Siddiqi Z, Kemper TL, Killiany R. Age-related neuronal loss from the substantia nigra-pars compacta and ventral tegmental area of the rhesus monkey. J Neuropathol Exp Neurol. 1999;58:959–71.
Wong KK, Muller ML, Kuwabara H, Studenski SA, Bohnen NI. Olfactory loss and nigrostriatal dopaminergic denervation in the elderly. Neurosci Lett. 2010;484:163–7.
Eckart C, Bunzeck N. Dopamine modulates processing speed in the human mesolimbic system. NeuroImage. 2013;66:293–300.
van Dyck CH, Avery RA, MacAvoy MG, Marek KL, Quinlan DM, Baldwin RM, et al. Striatal dopamine transporters correlate with simple reaction time in elderly subjects. Neurobiol Aging. 2008;29:1237–46.
Yang YK, Chiu NT, Chen CC, Chen M, Yeh TL, Lee IH. Correlation between fine motor activity and striatal dopamine D2 receptor density in patients with schizophrenia and healthy controls. Psychiatry Res. 2003;123:191–7.
Cham R, Perera S, Studenski SA, Bohnen NI. Striatal dopamine denervation and sensory integration for balance in middle-aged and older adults. Gait Posture. 2007;26:516–25.
Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res. 2008;185:391–8.
Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–212.
Dang LC, Samanez-Larkin GR, Castrellon JJ, Perkins SF, Cowan RL, Zald DH. Associations between dopamine D2 receptor availability and BMI depend on age. NeuroImage. 2016;138:176–83.
Castrellon JJ, Seaman KL, Crawford JL, Young JS, Smith CT, Dang LC, et al. Individual differences in dopamine are associated with reward discounting in clinical groups but not in healthy adults. J Neurosci. 2019;39:321–32.
Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, et al. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Ann Neurol. 1998;44:143–7.
Rudow G, O’Brien R, Savonenko AV, Resnick SM, Zonderman AB, Pletnikova O, et al. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol. 2008;115:461–70.
Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53:M20–6.
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N. Y Acad Sci. 2000;908:244–54.
Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69Suppl:S4–9.
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32.
Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26:1109–18.
Martinez-Cengotitabengoa M, Carrascon L, O’Brien JT, Diaz-Gutierrez MJ, Bermudez-Ampudia C, Sanada K, et al. Peripheral Inflammatory Parameters in Late-Life Depression: A Systematic Review. Int J Mol Sci. 2016;17:2022. https://doi.org/10.3390/ijms17122022.
Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–81.
Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–30.
Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC. Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry. 2014;22:1504–12.
Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78:720–7.
Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39:413–23.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34.
Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815.
Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–82.
Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33:315–27.
Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42:216–41.
Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–92.
Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69:1044–53.
Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–54.
Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–14.
Dipasquale O, Cooper EA, Tibble J, Voon V, Baglio F, Baselli G, et al. Interferon-alpha acutely impairs whole-brain functional connectivity network architecture - A preliminary study. Brain Behav Immun. 2016;58:31–9.
Keller AS, Leikauf JE, Holt-Gosselin B, Staveland BR, Williams LM. Paying attention to attention in depression. Transl Psychiatry. 2019;9:279.
Serra-Blasco M, Torres IJ, Vicent-Gil M, Goldberg X, Navarra-Ventura G, Aguilar E, et al. Discrepancy between objective and subjective cognition in major depressive disorder. Eur Neuropsychopharmacol. 2019;29:46–56.
Petersen JZ, Porter RJ, Miskowiak KW. Clinical characteristics associated with the discrepancy between subjective and objective cognitive impairment in depression. J Affect Disord. 2019;246:763–74.
DeJong H, Fox E, Stein A. Does rumination mediate the relationship between attentional control and symptoms of depression? J Behav Ther Exp Psychiatry. 2019;63:28–35.
Hendricks MA, Buchanan TW. Individual differences in cognitive control processes and their relationship to emotion regulation. Cogn Emot. 2016;30:912–24.
Gandelman JA, Albert K, Boyd BD, Park JW, Riddle M, Woodward ND, et al. Intrinsic functional network connectivity is associated with clinical symptoms and cognition in late-life depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:160–70.
Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–10.
Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–95.
Pimontel MA, Culang-Reinlieb ME, Morimoto SS, Sneed JR. Executive dysfunction and treatment response in late-life depression. Int J Geriatr Psychiatry. 2012;27:893–9.
Grahek I, Shenhav A, Musslick S, Krebs RM, Koster EHW. Motivation and cognitive control in depression. Neurosci Biobehav Rev. 2019;102:371–81.
Botvinick M, Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol. 2015;66:83–113.
Christman S, Bermudez C, Hao L, Landman BA, Boyd B, Albert K, et al. Accelerated brain aging predicts impaired cognitive performance and greater disability in geriatric but not midlife adult depression. Transl Psychiatry. 2020;10:317.
Respino M, Jaywant A, Kuceyeski A, Victoria LW, Hoptman MJ, Scult MA, et al. The impact of white matter hyperintensities on the structural connectome in late-life depression: Relationship to executive functions. Neuroimage Clin. 2019;23:101852.
Sheline YI, Barch DM, Garcia K, Gersing K, Piper C, Welsh-Bohmer KA, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58–65.
Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychological Med. 2000;30:679–91.
Lindenberger U, Mayr U, Kliegl R. Speed and intelligence in old age. Psychol Aging. 1993;8:207–20.
Sliwinski M, Buschke H. Processing speed and memory in aging and dementia. J Gerontol B Psychol Sci Soc Sci. 1997;52:P308–18.
Brown PJ, Liu X, Sneed JR, Pimontel MA, Devanand DP, Roose SP. Speed of processing and depression affect function in older adults with mild cognitive impairment. Am J Geriatr Psychiatry. 2013;21:675–84.
Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE). Am J Geriatr Psychiatry. 2005;13:134–41.
Backman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev. 2010;34:670–7.
Juarez EJ, Castrellon JJ, Green MA, Crawford JL, Seaman KL, Smith CT, et al. Reproducibility of the correlative triad among aging, dopamine receptor availability, and cognition. Psychol Aging. 2019;34:921–32.
Salami A, Garrett DD, Wahlin A, Rieckmann A, Papenberg G, Karalija N, et al. Dopamine D2/3 binding potential modulates neural signatures of working memory in a load-dependent fashion. J Neurosci. 2019;39:537–47.
Karlsson S, Nyberg L, Karlsson P, Fischer H, Thilers P, Macdonald S, et al. Modulation of striatal dopamine D1 binding by cognitive processing. NeuroImage. 2009;48:398–404.
Backman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C, et al. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000;157:635–7.
Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–9.
Backman L, Karlsson S, Fischer H, Karlsson P, Brehmer Y, Rieckmann A, et al. Dopamine D(1) receptors and age differences in brain activation during working memory. Neurobiol Aging. 2011;32:1849–56.
Erixon-Lindroth N, Farde L, Wahlin TB, Sovago J, Halldin C, Backman L. The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res. 2005;138:1–12.
Nyberg L, Karalija N, Salami A, Andersson M, Wahlin A, Kaboovand N, et al. Dopamine D2 receptor availability is linked to hippocampal-caudate functional connectivity and episodic memory. Proc Natl Acad Sci USA. 2016;113:7918–23.
Lovden M, Karalija N, Andersson M, Wahlin A, Axelsson J, Kohncke Y, et al. Latent-profile analysis reveals behavioral and brain correlates of dopamine-cognition associations. Cereb Cortex. 2018;28:3894–907.
Rutherford BR, Slifstein M, Chen C, Abi-Dargham A, Brown PJ, Wall MW, et al. Effects of L-DOPA monotherapy on psychomotor speed and [(11)C]raclopride binding in high-risk older adults with depression. Biol Psychiatry. 2019;86:221–9.
Roy MA, Doiron M, Talon-Croteau J, Dupre N, Simard M. Effects of antiparkinson medication on cognition in Parkinson’s disease: a systematic review. Can J Neurol Sci. 2018;45:375–404.
Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–72.
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405.
Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA. 2010;107:7036–41.
Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–8.
Wright CB, Sacco RL, Rundek T, Delman J, Rabbani L, Elkind M. Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. J Stroke Cerebrovasc Dis. 2006;15:34–8.
Heringa SM, van den Berg E, Reijmer YD, Nijpels G, Stehouwer CD, Schalkwijk CG, et al. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population - the Hoorn Study. Psychoneuroendocrinology. 2014;40:108–18.
Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195–204.
Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, Hack CE, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249–55.
Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423.
Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–81.
Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–8.
Kunisato Y, Okamoto Y, Ueda K, Onoda K, Okada G, Yoshimura S, et al. Effects of depression on reward-based decision making and variability of action in probabilistic learning. J Behav Ther Exp Psychiatry. 2012;43:1088–94.
Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35.
Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55.
Halahakoon DC, Kieslich K, O’Driscoll C, Nair A, Lewis G, Roiser JP. Reward-processing behavior in depressed participants relative to healthy volunteers: a systematic review and meta-analysis. JAMA Psychiatry. 2020;77:1286–95.
Husain M, Roiser JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci. 2018;19:470–84.
Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–56.
Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–85.
Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–45.
Suzuki S, Lawlor VM, Cooper JA, Arulpragasam AR, Treadway MT. Distinct regions of the striatum underlying effort, movement initiation and effort discounting. Nat Hum Behav. 2021;5:378–88.
Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, et al. Functional connectivity in apathy of late-life depression: a preliminary study. J Affect Disord. 2013;149:398–405.
Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360:815–36.
den Ouden HE, Kok P, de Lange FP. How prediction errors shape perception, attention, and motivation. Front Psychol. 2012;3:548.
Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–73.
Wise RA. Dopamine, learning, and motivation. Nat Rev Neurosci. 2004;5:483–94.
Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, et al. Mesolimbic dopamine signals the value of work. Nat Neurosci. 2016;19:117–26.
Howe MW, Tierney PL, Sandberg SG, Phillips PE, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013;500:575–9.
Berke JD. What does dopamine mean? Nat Neurosci. 2018;21:787–93.
du Hoffmann J, Nicola SM. Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci. 2014;34:14349–64.
Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology. 2007;191:507–20.
Satoh T, Nakai S, Sato T, Kimura M. Correlated coding of motivation and outcome of decision by dopamine neurons. J Neurosci. 2003;23:9913–23.
Castrellon JJ, Meade J, Greenwald L, Hurst K, Samanez-Larkin GR. Dopaminergic modulation of reward discounting in healthy rats: a systematic review and meta-analysis. Psychopharmacology. 2021;238:711–23.
Walton ME, Bouret S. What is the relationship between dopamine and effort? Trends Neurosci. 2019;42:79–91.
Tanaka S, O’Doherty JP, Sakagami M. The cost of obtaining rewards enhances the reward prediction error signal of midbrain dopamine neurons. Nat Commun. 2019;10:3674.
Huys QJ, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 2013;3:12.
Yang XH, Huang J, Zhu CY, Wang YF, Cheung EF, Chan RC, et al. Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 2014;220:874–82.
Clery-Melin ML, Schmidt L, Lafargue G, Baup N, Fossati P, Pessiglione M. Why don’t you try harder? An investigation of effort production in major depression. PLoS ONE. 2011;6:e23178.
Hershenberg R, Satterthwaite TD, Daldal A, Katchmar N, Moore TM, Kable JW, et al. Diminished effort on a progressive ratio task in both unipolar and bipolar depression. J Affect Disord. 2016;196:97–100.
Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87.
Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–45.
Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol Med. 2012;42:1203–15.
Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10:787–91.
Rademacher L, Salama A, Grunder G, Spreckelmeyer KN. Differential patterns of nucleus accumbens activation during anticipation of monetary and social reward in young and older adults. Soc Cogn Affect Neurosci. 2014;9:825–31.
Eppinger B, Hammerer D, Li SC. Neuromodulation of reward-based learning and decision making in human aging. Ann N. Y Acad Sci. 2011;1235:1–17.
Seaman KL, Brooks N, Karrer TM, Castrellon JJ, Perkins SF, Dang LC, et al. Subjective value representations during effort, probability and time discounting across adulthood. Soc Cogn Affect Neurosci. 2018;13:449–59.
Halfmann K, Hedgcock W, Kable J, Denburg NL. Individual differences in the neural signature of subjective value among older adults. Soc Cogn Affect Neurosci. 2016;11:1111–20.
Hess TM, Smith BT, Sharifian N. Aging and effort expenditure: the impact of subjective perceptions of task demands. Psychol Aging. 2016;31:653–60.
Devine ST, Neumann C, Otto AR, Bolenz F, Reiter AM, Eppinger B. Seizing the opportunity: Lifespan differences in the effects of the opportunity cost of time on cognitive control. Cognition. 2021;216:104863. https://doi.org/10.1016/j.cognition.2021.104863.
Manty M, de Leon CF, Rantanen T, Era P, Pedersen AN, Ekmann A, et al. Mobility-related fatigue, walking speed, and muscle strength in older people. J Gerontol A Biol Sci Med Sci. 2012;67:523–9.
Avlund K, Rantanen T, Schroll M. Tiredness and subsequent disability in older adults: the role of walking limitations. J Gerontol A Biol Sci Med Sci. 2006;61:1201–5.
Salamone JD, Correa M, Yohn S, Lopez Cruz L, San Miguel N, Alatorre L. The pharmacology of effort-related choice behavior: dopamine, depression, and individual differences. Behav Process. 2016;127:3–17.
Robles CF, Johnson AW. Disruptions in effort-based decision-making and consummatory behavior following antagonism of the dopamine D2 receptor. Behav Brain Res. 2017;320:431–9.
Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci. 2011;31:16597–602.
Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6.
Caravaggio F, Fervaha G, Browne CJ, Gerretsen P, Remington G, Graff-Guerrero A. Reward motivation in humans and its relationship to dopamine D2/3 receptor availability: A pilot study with dual [(11)C]-raclopride and [(11)C]-(+)-PHNO imaging. J Psychopharmacol. 2018;32:357–66.
Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7.
Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–5.
Chowdhury R, Guitart-Masip M, Lambert C, Dayan P, Huys Q, Duzel E, et al. Dopamine restores reward prediction errors in old age. Nat Neurosci. 2013;16:648–53.
Beierholm U, Guitart-Masip M, Economides M, Chowdhury R, Duzel E, Dolan R, et al. Dopamine modulates reward-related vigor. Neuropsychopharmacology. 2013;38:1495–503.
Michely J, Viswanathan S, Hauser TU, Delker L, Dolan RJ, Grefkes C. The role of dopamine in dynamic effort-reward integration. Neuropsychopharmacology. 2020;45:1448–53.
Hofmans L, Papadopetraki D, van den Bosch R, Maatta JI, Frobose MI, Zandbelt BB, et al. Methylphenidate boosts choices of mental labor over leisure depending on striatal dopamine synthesis capacity. Neuropsychopharmacology. 2020;45:2170–9.
Westbrook A, van den Bosch R, Maatta JI, Hofmans L, Papadopetraki D, Cools R, et al. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science. 2020;367:1362–6.
Sharp ME, Foerde K, Daw ND, Shohamy D. Dopamine selectively remediates ‘model-based’ reward learning: a computational approach. Brain. 2016;139:355–64.
Foerde K, Figner B, Doll BB, Woyke IC, Braun EK, Weber EU, et al. Dopamine modulation of intertemporal decision-making: evidence from Parkinson disease. J Cogn Neurosci. 2016;28:657–67.
Chong TT, Bonnelle V, Manohar S, Veromann KR, Muhammed K, Tofaris GK, et al. Dopamine enhances willingness to exert effort for reward in Parkinson’s disease. Cortex. 2015;69:40–6.
Muhammed K, Manohar S, Ben Yehuda M, Chong TT, Tofaris G, Lennox G, et al. Reward sensitivity deficits modulated by dopamine are associated with apathy in Parkinson’s disease. Brain. 2016;139:2706–21.
Panigrahi B, Martin KA, Li Y, Graves AR, Vollmer A, Olson L, et al. Dopamine is required for the neural representation and control of movement vigor. Cell. 2015;162:1418–30.
Liu Y, Admon R, Mellem MS, Belleau EL, Kaiser RH, Clegg R, et al. Machine learning identifies large-scale reward-related activity modulated by dopaminergic enhancement in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:163–72.
Rengasamy M, Marsland A, McClain L, Kovats T, Walko T, Pan L, et al. Longitudinal relationships of cytokines, depression and anhedonia in depressed adolescents. Brain Behav Immun. 2021;91:74–80.
Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2020;25:1301–11.
Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70:31–41.
Lee Y, Mansur RB, Brietzke E, Carmona NE, Subramaniapillai M, Pan Z, et al. Efficacy of adjunctive infliximab vs. placebo in the treatment of anhedonia in bipolar I/II depression. Brain Behav Immun. 2020;88:631–9.
Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–65.
Yin L, Xu X, Chen G, Mehta ND, Haroon E, Miller AH, et al. Inflammation and decreased functional connectivity in a widely-distributed network in depression: Centralized effects in the ventral medial prefrontal cortex. Brain Behav Immun. 2019;80:657–66.
Burrows K, Stewart JL, Kuplicki R, Figueroa-Hall L, Spechler PA, Zheng H, et al. Elevated peripheral inflammation is associated with attenuated striatal reward anticipation in major depressive disorder. Brain Behav Immun. 2021;93:214–25.
Seidler RD, Alberts JL, Stelmach GE. Changes in multi-joint performance with age. Mot Control. 2002;6:19–31.
Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. Jama. 1989;261:2663–8.
Karpman C, DePew ZS, LeBrasseur NK, Novotny PJ, Benzo RP. Determinants of gait speed in COPD. Chest. 2014;146:104–10.
Dawson J, Linsell L, Zondervan K, Rose P, Randall T, Carr A, et al. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology. 2004;43:497–504.
Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901.
Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31.
Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–7.
Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–9.
White DK, Neogi T, Nevitt MC, Peloquin CE, Zhu Y, Boudreau RM, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2013;68:456–64.
Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, Camera LA, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–9.
Demakakos P, Cooper R, Hamer M, de Oliveira C, Hardy R, Breeze E. The bidirectional association between depressive symptoms and gait speed: evidence from the English Longitudinal Study of Ageing (ELSA). PLoS ONE. 2013;8:e68632.
Sanders JB, Bremmer MA, Deeg DJ, Beekman AT. Do depressive symptoms and gait speed impairment predict each other’s incidence? A 16-year prospective study in the community. J Am Geriatr Soc. 2012;60:1673–80.
Stahl ST, Altmann HM, Dew MA, Albert SM, Butters M, Gildengers A, et al. The effects of gait speed and psychomotor speed on risk for depression and anxiety in older adults with medical comorbidities. J Am Geriatr Soc. 2021;69:1265–71.
Rosario BL, Rosso AL, Aizenstein HJ, Harris T, Newman AB, Satterfield S, et al. Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J Gerontol A Biol Sci Med Sci. 2016;71:968–73.
Sanders JB, Bremmer MA, Comijs HC, van de Ven PM, Deeg DJH, Beekman ATF. Gait speed and processing speed as clinical markers for geriatric health outcomes. Am J Geriatr Psychiatry. 2017;25:374–85.
Brown PJ, Roose SP, Zhang J, Wall M, Rutherford BR, Ayonayon HN, et al. Inflammation, depression, and slow gait: a high mortality phenotype in later life. J Gerontol A Biol Sci Med Sci. 2016;71:221–7.
Cham R, Perera S, Studenski SA, Bohnen NI. Age-related striatal dopaminergic denervation and severity of a slip perturbation. J Gerontol A Biol Sci Med Sci. 2011;66:980–5.
Bohnen NI, Muller ML, Kuwabara H, Cham R, Constantine GM, Studenski SA. Age-associated striatal dopaminergic denervation and falls in community-dwelling subjects. J Rehabil Res Dev. 2009;46:1045–52.
Calvani R, Marini F, Cesari M, Buford TW, Manini TM, Pahor M, et al. Systemic inflammation, body composition, and physical performance in old community-dwellers. J Cachexia Sarcopenia Muscle. 2017;8:69–77.
Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–13.
Verghese J, Holtzer R, Oh-Park M, Derby CA, Lipton RB, Wang C. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1083–9.
Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–80.
Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10.
Verghese J, Holtzer R, Lipton RB, Wang C. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing. 2012;41:541–5.
Custodero C, Anton SD, Beavers DP, Mankowski RT, Lee SA, McDermott MM, et al. The relationship between interleukin-6 levels and physical performance in mobility-limited older adults with chronic low-grade inflammation: The ENRGISE Pilot study. Arch Gerontol Geriatr. 2020;90:104131.
Rong YD, Bian AL, Hu HY, Ma Y, Zhou XZ. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018;18:308.
Westbrook A, Kester D, Braver TS. What is the subjective cost of cognitive effort? Load, trait, and aging effects revealed by economic preference. PLoS ONE. 2013;8:e68210.
Yee DM, Adams S, Beck A, Braver TS. Age-related differences in motivational integration and cognitive control. Cogn Affect Behav Neurosci. 2019;19:692–714.
Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cogn Affect Behav Neurosci. 2008;8:99–112.
Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–89.
Camicioli R, Wang Y, Powell C, Mitnitski A, Rockwood K. Gait and posture impairment, parkinsonism and cognitive decline in older people. J Neural Transm. 2007;114:1355–61.
Thompson PD. Gait disorders accompanying diseases of the frontal lobes. Adv Neurol. 2001;87:235–41.
Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–23.
Belur P, Hsiao D, Myers PS, Earhart GM, Rawson KS. Dual-task costs of texting while walking forward and backward are greater for older adults than younger adults. Hum Mov Sci. 2020;71:102619.
Galaro JK, Celnik P, Chib VS. Motor cortex excitability reflects the subjective value of reward and mediates its effects on incentive-motivated performance. J Neurosci. 2019;39:1236–48.
Summa S, Tamagnone I, Asprea G, Capurro C, Sanguineti V. Modulation of motor performance by a monetary incentive: a pilot study. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:238–41.
Cusin C, Iovieno N, Iosifescu DV, Nierenberg AA, Fava M, Rush AJ, et al. A randomized, double-blind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J Clin Psychiatry. 2013;74:e636–41.
Gershon AA, Amiaz R, Shem-David H, Grunhaus L. Ropinirole augmentation for depression: a randomized controlled trial pilot study. J Clin Psychopharmacol. 2019;39:78–81.
Lavretsky H, Reinlieb M, St Cyr N, Siddarth P, Ercoli LM, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172:561–9.
Alexopoulos GS, Raue PJ, Banerjee S, Marino P, Renn BN, Solomonov N, et al. Comparing the streamlined psychotherapy “Engage” with problem-solving therapy in late-life major depression. A randomized clinical trial. Mol Psychiatry. 2020. https://doi.org/10.1038/s41380-020-0832-3.
Morimoto SS, Wexler BE, Liu J, Hu W, Seirup J, Alexopoulos GS. Neuroplasticity-based computerized cognitive remediation for treatment-resistant geriatric depression. Nat Commun. 2014;5:4579.
Zecca L, Tampellini D, Gerlach M, Riederer P, Fariello RG, Sulzer D. Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Mol Pathol. 2001;54:414–8.
Liang CL, Nelson O, Yazdani U, Pasbakhsh P, German DC. Inverse relationship between the contents of neuromelanin pigment and the vesicular monoamine transporter-2: human midbrain dopamine neurons. J Comp Neurol. 2004;473:97–106.
Shibata E, Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S, et al. Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magn Reson Med Sci. 2006;5:197–200.
Castellanos G, Fernandez-Seara MA, Lorenzo-Betancor O, Ortega-Cubero S, Puigvert M, Uranga J, et al. Automated neuromelanin imaging as a diagnostic biomarker for Parkinson’s disease. Mov Disord. 2015;30:945–52.
Kawaguchi H, Shimada H, Kodaka F, Suzuki M, Shinotoh H, Hirano S, et al. Principal component analysis of multimodal neuromelanin MRI and dopamine transporter PET data provides a specific metric for the nigral dopaminergic neuronal density. PLoS ONE. 2016;11:e0151191.
Cassidy CM, Zucca FA, Girgis RR, Baker SC, Weinstein JJ, Sharp ME, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci USA. 2019;116:5108–17.
Acknowledgements
This research was supported by National Institute of Health grants K24 MH110598, R01 MH123660 and R01 MH123662. WDT would additionally like to acknowledge salary support from the Tennessee Valley Healthcare System Geriatric Research Education and Clinical Center (GRECC).
Author information
Authors and Affiliations
Contributions
WDT, DHZ, JCF, SC, SMS and BRR drafted the manuscript, while WDT, DHZ, JCF, DOC, GH, JMM, KG, BRR contributed to the conceptualization, scientific model, integration of past literature, and critical revisions of the intellectual content. All authors approved the manuscript, with WDT, DHZ and BRR providing final approval for the published version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taylor, W.D., Zald, D.H., Felger, J.C. et al. Influences of dopaminergic system dysfunction on late-life depression. Mol Psychiatry 27, 180–191 (2022). https://doi.org/10.1038/s41380-021-01265-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01265-0
This article is cited by
-
Biological factors influencing depression in later life: role of aging processes and treatment implications
Translational Psychiatry (2023)
-
Brain-cognition relationships in late-life depression: a systematic review of structural magnetic resonance imaging studies
Translational Psychiatry (2023)
-
Preservation of dendritic D2 receptor transmission in substantia nigra dopamine neurons with age
Scientific Reports (2023)
-
Sex matters: acute functional connectivity changes as markers of remission in late-life depression differ by sex
Molecular Psychiatry (2023)
-
Depression im Alter und Frailty – epidemiologische, klinische und neurobiologische Zusammenhänge
Der Nervenarzt (2023)