Summary
Abstract
Amisulpride (Solian®), a substituted benzamide derivative, is a second-generation antipsychotic that preferentially binds to dopamine D2/D3 receptors in limbic rather than striatal structures. High dosages preferentially antagonise postsynaptic D2/D3 receptors, resulting in reduced dopamine transmission, and low dosages preferentially block presynaptic D2/D3 receptors, resulting in enhanced dopamine transmission.
Amisulpride (200–1200 mg/day) was at least as effective as haloperidol and as effective as risperidone or olanzapine, in studies of up to 1 year in patients with schizophrenia manifesting predominantly positive symptoms. Amisulpride (50–300 mg/day) was significantly more effective than placebo in studies of up to 6 months in patients manifesting predominantly negative symptoms. Quality of life was also improved significantly more in patients receiving amisulpride than in those receiving haloperidol in 4- and 12-month studies in patients with predominantly mixed symptoms.
Amisulpride was generally well tolerated in clinical trials. In patients with predominantly positive symptoms, amisulpride appeared to be better tolerated than haloperidol and was tolerated as well as risperidone and olanzapine. The incidence of extrapyramidal adverse effects with amisulpride was lower than with haloperidol but was generally similar to risperidone or olanzapine. Weight gain with amisulpride was less than that with risperidone or olanzapine and, unlike these agents, amisulpride does not seem to be associated with diabetogenic effects. Plasma prolactin levels are increased during amisulpride therapy and amenorrhoea occurs in about 4% of women. The incidence of adverse events with low dosages of amisulpride (≤300 mg/day) in patients with predominantly negative symptoms was similar to that observed with placebo.
In conclusion, oral amisulpride (200–1200 mg/day) is at least as effective as haloperidol, and as effective as risperidone or olanzapine, in the treatment of patients with schizophrenia manifesting predominantly positive symptoms. In the treatment of patients manifesting predominantly negative symptoms, low dosages of amisulpride (50–300 mg/day) are significantly more effective than placebo. Amisulpride appears to be better tolerated than haloperidol, causing a lower incidence of extrapyramidal adverse effects and an improved quality of life. Compared with risperidone or olanzapine, amisulpride is more likely to cause hyperprolactinaemia, but has a lower propensity to cause weight gain and does not seem to be associated with diabetogenic effects. Thus, amisulpride is an effective and well tolerated option for the first-line treatment of patients with acute schizophrenia as well as for those requiring long-term maintenance therapy.
Pharmacoloaical Properties
Amisulpride has selective affinity for human D2 and D3 receptor subtypes, no affinity for D1, D4 and D5 receptor subtypes and little affinity for the adrenergic, histaminergic, serotonergic or cholinergic receptors. Amisulpride preferentially binds to D2/D3 receptors in limbic rather than striatal structures. Low dosages (<10 mg/kg) preferentially block presynaptic D2/D3 receptors, resulting in enhanced dopamine transmission; higher dosages preferentially antagonise post-synaptic D2/D3 receptors, resulting in reduced dopamine transmission.
Unlike haloperidol, amisulpride does not appear to be associated with impaired cognitive function. Plasma prolactin concentrations are increased, particularly in the early weeks of amisulpride therapy.
Two absorption peaks are demonstrated after a single oral dose of amisulpride 50mg in healthy volunteers; peak plasma concentrations of 42 and 56 µg/L were recorded at hours 1 and 4. The absolute bioavailability is approximately 50% and the volume of distribution is 5.8 L/kg. Only minimal amounts of amisulpride are bound to plasma proteins (17%). The potential for drug interactions is low and amisulpride does not appear to affect the activity of the cytochrome P450 system.
Amisulpride undergoes minimal metabolism and is primarily excreted in the urine. The pharmacokinetics of amisulpride were unchanged after repeated administration of 200 mg/day for 7 days. Elimination is biphasic after oral administration with a plasma elimination half-life of approximately 12 hours. Renal clearance is about 20 L/h in healthy volunteers.
Clinical Efficacy
In well designed trials of 6–52 weeks’ duration in patients with schizophrenia manifesting predominantly positive symptoms, amisulpride (200–1200 mg/day) was at least as effective in the control of positive symptoms as haloperidol (5–30 mg/day), but appeared more effective than haloperidol in the control of negativesymptoms. In this patient population, amisulpride was as effective as the atypical agents risperidone (4–10 mg/day) and olanzapine (5–20 mg/day). For some parameters (e.g. percentage of patients with ≥50% improvement in Positive and Negative Syndrome Scale and Brief Psychiatric Rating Scale at 6 months), results were more favourable with amisulpride than with risperidone in a double-blind, randomised trial.
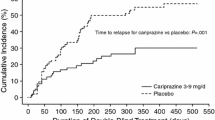
In patients manifesting predominantly negative symptoms of schizophrenia, low dosages of amisulpride (50–300 mg/day) were significantly more effective than placebo in improving symptoms, as assessed by the Scale for the Assessment of Negative Symptoms in three double-blind studies of up to 24 weeks’ duration.
Quality-of-life measures were improved more with amisulpride than with haloperidol in patients with predominantly mixed symptoms.
Tolerability
Amisulpride is generally well tolerated at high and low dosages. In clinical trials in patients with acute exacerbations of schizophrenia manifesting predominantly positive symptoms, the incidence of adverse events was similar with amisulpride (200–1200 mg/day), haloperidol (5–30 mg/day), risperidone (4–10 mg/day) and olanzapine (5–20 mg/day), but the tolerability profiles differed. The most common adverse events associated with amisulpride therapy in a pooled analysis of 11 comparative trials were extrapyramidal disorders (15%), insomnia (11%), hyperkinesia (9%), anxiety (9%), bodyweight increase (7%) and agitation (6%). In patients with predominantly negative symptoms, the incidence of adverse events was similar with amisulpride (50–300 mg/day) or placebo.
The incidence of extrapyramidal adverse events associated with amisulpride 200–1200 mg/day was markedly lower than that with haloperidol, but was generally similar to that of atypical agents (risperidone and olanzapine). Bodyweight gain was less with amisulpride than with risperidone or olanzapine and, unlike these agents, amisulpride does not seem to be associated with diabetogenic effects.
Elevated plasma prolactin levels can occur with antipsychotic agents as a result of their D2 receptor antagonism, and hyperprolactinaemia may result in both acute (e.g. amenorrhoea, galactorrhoea, reduced libido) and chronic (predisposition to osteoporosis and cardiovascular disease) effects. Plasma prolactin levels are increased during amisulpride therapy, but the incidence of endocrine disorders is low. In a pooled analysis, amisulpride caused a higher incidence of amenorrhoea (4% of female patients) than haloperidol (0%) or risperidone (0%), although the difference was not statistically significant. Similarly, amenorrhoea was reported in 6% and 0% of women treated with amisulpride and olanzapine, respectively, in a 6-month randomised trial.
Consistent with the lack of effect on the α-adrenergic and cholinergic receptors, amisulpride does not appear to cause clinically relevant changes to the QT interval, heart rate or blood pressure.
Dosage and Administration
The recommended dosage of amisulpride for acute psychotic episodes in patients with schizophrenia is 400–800 mg/day orally. The dosage may be increased up to 1200 mg/day if necessary. Maintenance therapy should be individualised with the minimally effective dosage. For patients with predominantly negative symptoms of schizophrenia, dosages of 50–300 mg/day are recommended.










Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Feetam C, Donoghue J. Antipsychotics in the treatment of first-episode schizophrenia. Pharm J 2003 Mar 22; 270(7241): 405–8
Kasper S. Dopaminergic deficit and the role of amisulpride in the treatment of schizophrenia. Int Clin Psychopharmacol 2002 Dec; 17Suppl 4: S19–26
Kapur S, Remington G. Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med 2001; 52: 503–17
Tarsy D, Baldessarini RJ, Tarazi FI. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 2002; 16(1): 23–45
Coukell AJ, Spencer CM, Benfield P. Amisulpride: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of schizophrenia. CNS Drugs 1996 Sep; 6(3): 237–56
Scatton B, Claustre Y, Cudennec A, et al. Amisulpride: from animal pharmacology to therapeutic action. Int Clin Psychopharmacol 1997 May; 12Suppl 2: S29–36
Perrault GH, Depoortere R, Morel E, et al. Psychopharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther 1997; 280(1): 73–82
Schoemaker H, Claustre Y, Fage D, et al. Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynaptic and limbic selectivity. J Pharmacol Exp Ther 1997; 280: 83–97
Schotte A, Janssen PFM, Gommeren W, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996; 124: 57–73
Nordström A-L, Farde L, Nyberg S, et al. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry 1995; 152: 1444–9
Möller HJ. Amisulpride: limbic specificity and the mechanism of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry 2003 Oct; 27(7): 1101–11
Cudennec A, Fage D, Bénavidès J, et al. Effects of amisulpride, an atypical antipsychotic which blocks preferentially presynaptic dopamine autoreceptors, on integrated functional cerebral activity in the rat. Brain Res 1997; 768: 257–65
Bressan RA, Erlandsson K, Jones HM, et al. Is regionally selective D2/D3 dopamine occupancy sufficient for atypical antipsychotic effect? An in vivo quantitative [123I]epidepride SPET study of amisulpride-treated patients. Am J Psychiatry 2003 Aug; 160(8): 1413–20
Martinot JL, Paillere-Martinot ML, Poirier MF, et al. In vivo characteristics of dopamine D2 receptor occupancy by amisulpride in schizophrenia. Psychopharmacology (Berl) 1996; 124: 154–8
Farde L, Nordström A-L, Wiesel F-A, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine: relation to extrapyramidal side effects. Arch Gen Psychiatry 1992 Jul; 49: 538–44
Farde L, Nordström A-L, Karlsson P, et al. Positron emission tomography studies on dopamine receptors in schizophrenia. Clin Neuropharmacol 1995; 18Suppl. 1: S121–9
Xiberas X, Martinot J-L, Mallet L, et al. In vivo extrastriatal and striatal D2 dopamine receptor blockade by amisulpride in schizophrenia. J Clin Psychopharmacol 2001; 21(2): 207–14
Trichard C, Paillère-Martinot M-L, Attar-Levy D, et al. Binding of antipsychotic drugs to cortical 5-HT2A receptors: a PET study of chlorpromazine, clozapine, and amisulpride in schizophrenic patients. Am J Psychiatry 1998; 155: 505–8
Fric M, Laux G. Prolactin levels and symptoms of hyperprolactinemia in patients treated with amisulpride, risperidone, olanzapine and quetiapine [in German]. Psychiatr Prax 2003; 30Suppl. 2: S97–101
Kapur S, Langlois X, Vinken P, et al. The differential effects of atypical antipsychotics on prolactin elevation are explained by their differential blood-brain disposition: a pharmacological analysis in rats. J Pharmacol Exp Ther 2002 Sep; 302(3): 1129–34
Kopecek M, Bares M, Svarc J, et al. Hyperprolactinemia after low dose of amisulpride [abstract no. P01.186]. 24th Congress of the Collegium Internationale Neuro-Psychopharmacologicum; 2004 Jun 20–24; Paris
Wetzel H, Wiesner J, Hiemke C, et al. Acute antagonism of dopamine D2-like receptors by amisulpride: effects on hormone secretion in healthy volunteers. J Psychiatr Res 1994; 28(5): 461–73
Ramaekers JG, Louwerens JW, Muntjewerff ND, et al. Psychomotor, cognitive, extrapyramidal, and affective functions of healthy volunteers during treatment with an atypical (amisulpride) and a classic (haloperidol) antipsychotic. J Clin Psychopharmacol 1999; 19(3): 209–21
Peretti CS, Danion JM, Kauffmann-Muller F, et al. Effects of haloperidol and amisulpride on motor and cognitive skill learning in healthy volunteers. Psychopharmacology (Berl) 1997; 131: 329–38
Legangneux E, McEwen J, Wesnes KA, et al. The acute effects of amisulpride (50 mg and 200 mg) and haloperidol (2 mg) on cognitive function in healthy elderly volunteers. J Psychopharmacol (Oxf) 2000; 14(2): 164–71
Perault MC, Bergougnan L, Paillat A, et al. Lack of interaction between amisulpride and lorazepam on psychomotor performance and memory in healthy volunteers. Human Psychopharmacol 1998; 13(7): 493–500
Mattila MJ, Patat A, Seppälä T, et al. Single oral doses of amisulpride do not enhance the effects of alcohol on the performance and memory of healthy subjects. Eur J Clin Pharmacol 1996; 51: 161–6
Patat A, Rosenzweig P, Miget N, et al. Effects of 50mg amisulpride on EEG, psychomotor and cognitive functions in healthy sleep-deprived subjects. Fundam Clin Pharmacol 1999; 13: 582–94
Vaiva G, Thomas P, Llorca PM, et al. SPECT imaging, clinical features, and cognition before and after low doses of amisulpride in schizophrenic patients with the deficit syndrome. Psychiatry Res Neuroimaging 2002 Aug 20; 115(1–2): 37–48
Adler G, Fraude V, Thebaldi B, et al. Cognitive performance and side effects under clozapine and amisulpride treatment in patients with chronic schizophrenia [abstract no. P01.427]. 24th Congress of the Collegium Internationale Neuro-Psychopharmacologicum; 2004 Jun 20–24; Paris
Joyce E, Rein W, Fleurot O. Effect of amisulpride and olanzapine on neuropsychological performance in schizophrenic patients: preliminary results from a double-blind, randomised trial. 24th Congress of the Collegium Internationale Neuro-Psychopharmacologicum; 2004 Jun 20–24; Paris
Curran MP, Perry CM. Amisulpride: a review of its use in the management of schizophrenia. Drugs 2001; 61(14): 2123–50
Rosenzweig P, Canal M, Patat A, et al. A review of the pharmacokinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers. Hum Psychopharmacol 2002 Jan; 17(1): 1–13
Dufour A, Desanti C. Pharmacokinetics and metabolism of amisulpride [in French]. Annales De Psychiatrie 1988; 3(3): 298–305
Sanofi Synthelabo. Solian: summary of product characteristics [online]. Available from URL: http://emc.medicines.org.uk [Accessed 2004 Mar 17]
Canal M, Legangneux E, Van Lier JJ, et al. Lack of effect of amisulpride on the pharmacokinetics and safety of lithium. Int J Neuropsychopharmacol 2003 Jun; 6(2): 103–9
American Psychiatric Association. Practice guidelines for the treatment of patients with schizophrenia: second edition [online]. Available from URL: http://www.psych.org [Accessed 2004 Mar 25]
Boyer P, Lecrubier Y, Puech AJ, et al. Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry 1995; 166: 68–72
Loo H, Poirier-Littre M-F, Theron M, et al. Amisulpride versus placebo in the medium-term treatment of the negative symptoms of schizophrenia. Br J Psychiatry 1997; 170: 18–22
Speller JC, Barnes TRE, Curson DA, et al. One-year, low-dose neuroleptic study of in-patients with chronic schizophrenia characterised by persistent negative symptoms: amisulpride v. haloperidol. Br J Psychiatry 1997; 171: 564–8
Colonna L, Saleem P, Dondey-Nouvel L, et al. Long-term safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Amisulpride Study Group. Int Clin Psychopharmacol 2000; 15(1): 13–22
Möller HJ, Boyer P, Fleurot O, et al. Improvement of acute exacerbations of schizophrenia with amisulpride: a comparison with haloperidol. PROD-ASLP Study Group. Psychopharmacology (Berl) 1997; 132: 396–401
Danion J-M, Rein W, Fleurot O. Improvement of schizophrenic patients with primary negative symptoms treated with amisulpride. Amisulpride Study Group. Am J Psychiatry 1999; 156: 610–6
Sechter D, Peuskens J, Fleurot O, et al. Amisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropsychopharmacology 2002 Dec; 27(6): 1071–81
Peuskens J, Bech P, Moller H-J, et al. Amisulpride vs. risperidone in the treatment of acute exacerbations of schizophrenia. Amisulpride Study Group. Psychiatry Res 1999; 88: 107–17
Carrière P, Bonhomme D, Lempérière T. Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre, double-blind study. Amisulpride Study Group. Eur Psychiatry 2000 Aug; 15(5): 321–9
Mortimer A, Martin S, Lôo H, et al. A double-blind, randomized comparative trial of amisulpride versus olanzapine for 6 months in the treatment of schizophrenia. Int Clin Psychopharmacol 2004; 19(2): 63–9
Vanelle JM, Douki S. An 8-week double blind, randomised trial comparing amisulpride and olanzapine in schizophrenic patients with depression. Schizodep Study Group [abstract plus poster no. P01.425]. 24th Congress of the Collegium Internationale Neuro-Psychopharmacologicum; 2004 Jun 20–24; Paris
Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003 Jun; 60(6): 553–64
Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ 2000 Dec 2; 321(7273): 1371–6
Leucht S, Pitschel-Walz G, Engel RR, et al. Amisulpride, an unusual “atypical” antipsychotic: a meta-analysis of randomized controlled trials. Am J Psychiatry 2002 Feb; 159(2): 180–90
Rein W, Fleurot O. Efficacy of amisulpride vs risperidone in the long-term treatment of chronic schizophrenia: results from a 12 month double-blind study [abstract no. P.2.113]. Eur Neuropsychopharmacol 2002 Oct; 12Suppl. 3: S302
Peuskens J, Möller HJ, Puech A. Amisulpride improves depressive symptoms in acute exacerbations of schizophrenia: comparison with haloperidol and risperidone. Eur Neuropsychopharmacol 2002 Aug; 12(4): 305–10
The European Agency for the Evaluation of Medicinal Products. Note for guidance on the clinical investigation of medicinal products in the treatment of schizophrenia [online]. Available from URL: http://www.emea.eu.int [Accessed 2004 Apr 5]
Coulouvrat C, Dondey-Nouvel L. Safety of amisulpride (Solian®): a review of 11 clinical studies. Int Clin Psychopharmacol 1999; 14: 209–18
Müller MJ, Sachse J, Vernaleken I, et al. Therapeutic drug monitoring of amisulpride and clinical outcome [abstract no. 149]. Ther Drug Monit 2003 Aug; 25(4): 523
Mir S, Taylor D. Atypical antipsychotics and hyperglycaemia. Int Clin Psychopharmacol 2001; 16: 63–74
Koller E, Schneider B, Bennett K, et al. Clozapine-associated diabetes. Am J Med 2001; 111: 716–23
Koller EA, Doraiswamy PM. Olanzapine-associated diabetes mellitus. Pharmacotherapy 2002; 22(7): 841–52
Koller EA, Cross JT, Doraiswamy PM, et al. Risperidone-associated diabetes mellitus: a pharmacovigilance study. Pharmacotherapy 2003; 23(6): 735–44
Koller EA, Doraiswamy PM, Cross JT, et al. Quetiapine-associated diabetes mellitus [abstract no. NR741]. 156th Annual Meeting of the American Psychiatric Association; 2003 May 17–22; San Francisco, 277
Leucht S, Wagenpfeil S, Hamann J, et al. Amisulpride is an “atypical” antipsychotic associated with low weight gain. Psychopharmacology (Berl) 2004; 173: 112–5
Agelink MW, Majewski T, Wurthmann C, et al. Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. J Clin Psychopharmacol 2001; 21(1): 8–13
Peuskens J, Lancrenon S, Rein W. Metabolic control in schizophrenic patients treated with atypical antipsychotics: a subanalysis of a comparative trial of amisulpride and olanzapine [abstract no. P02.434]. 24th Congress of the Collegium Internationale Neuro-Psychopharmacologicum; 2004 Jun 20–24; Paris
Sartorius M, Fleischhacker WW, Gjerris A, et al. The usefulness and use of second-generation antipsychotic medications. Curr Opin Psychiatry 2002; 15Suppl. 1: S1–51
Souetre E, Martin P, Lecanu JP, et al. Economic assessment of neuroleptic strategies in schizophrenia [in French]. Encephale 1992; 18: 263–9
Nicholls CJ, Hale AS, Freemantle N. Cost-effectiveness of amisulpride compared with risperidone in patients with schizophrenia. J Drug Assess 2003; 6(2): 79–89
Sanofi-Synthelabo Australia Pty Limited. Product information: Solian. North Ryde: Sanofi-Synthelabo, 2004 Feb
Burns T, Chabannes JP, Demyttenaere K. Switching antipsychotic medications: general recommendations and switching to amisulpride. Curr Med Res Opin 2002; 18(4): 201–8
National Institute for Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in primary and secondary care. Clinical Guideline. London: NICE, 2002 Dec: 1–62
Kerwin R. From pharmacological profiles to clinical outcomes. Int Clin Psychopharmacol 2000; 15Suppl. 4: S1–4
Hummer M, Huber J. Hyperprolactinaemia and antipsychotic therapy in schizophrenia. Curr Med Res Opin 2004; 20(2): 189–97
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: C. Feetam, Department of Psychiatric Pharmacy, Aston University, Birmingham, England; S.W. Lewis, Neuroscience and Psychiatry Unit, University of Manchester, Manchester, England; H.-J. Möller, Psychiatric Department, University of Munich, Munich, Germany; J. Peuskens, Psychiatrisch Instituut, Universitair Centrum St Jozef, Kortenberg, Belgium; S.P. Singh, Department of Mental Health, St George’s Hospital Medical School, London, England.
Data Selection
Sources: Medical literature published in any language since 1980 on amisulpride, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘amisulpride ’ and ‘schizophrenia ’ and ‘amisulpride ’ and ( ‘pharmacodynamics ’ or ‘pharmacokinetics ’). EMBASE search terms were ‘amisulpride ’ and ‘schizophrenia ’. AdisBase search terms were ‘amisulpride ’ and ‘schizophrenia ’ and ‘amisulpride ’ and ( ‘PD ’ or ‘PK ’). Searches were last updated 20 September 2004.
Selection: Studies in patients with schizophrenia who received amisulpride. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Amisulpride, antipsychotics, schizophrenia, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
McKeage, K., Plosker, G.L. Amisulpride. CNS Drugs 18, 933–956 (2004). https://doi.org/10.2165/00023210-200418130-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200418130-00007