Abstract
Functional neurological symptom disorder (FNSD) is characterized by neurological symptoms that are unexplained by other traditional neurological or medical conditions. Both physicians and patients have limited understanding of FNSD, which is often explained as a physical manifestation of psychological distress. Recently, diagnostic criteria have shifted from requiring a preceding stressor to relying on positive symptoms. Given this shift, we have provided a review of the etiology of FNSD. Predisposing factors include trauma or psychiatric symptoms, somatic symptoms, illness exposure, symptom monitoring and neurobiological factors. Neurobiological research has indicated that patients with FNSD have a decreased sense of agency and abnormal attentional focus on the affected area, both of which are modulated by beliefs and expectations about illness. Sick role and secondary gain may reinforce and maintain FNSD. The integrated etiological summary model combines research from various fields and other recent etiological models to represent the current understanding of FNSD etiology. It discusses a potential causal mechanism and informs future research and treatment.
Introduction
Functional neurological symptom disorder (FNSD) refers to neurological symptoms that are incompatible with neurological or medical conditions.1 The incidence is between 4 and 12 per 100 000,2 comparable to multiple sclerosis and amyotrophic lateral sclerosis,3 and it is the second most common diagnosis in neurology clinics. Examples of FNSD include psychogenic nonepileptic seizures (PNES), paralysis, functional movement disorders (FMD), blindness and non-dermatomal sensory deficits.1
The prognosis for FNSD is linked to early diagnosis and symptom duration,4,5 but the average time to diagnosis of PNES is more than 7 years.6 Delayed diagnosis and unnecessary medications can lead to iatrogenic effects, delay appropriate treatment and negatively affect prognosis.6,7 However, both physicians and patients have a limited understanding of FNSD. One study demonstrated that physicians held several misperceptions about PNES, and that their confidence in their ability to treat PNES was low.8 This uncertainty likely affects patients with FNSD. Most patients with PNES do not have a good understanding of their diagnosis, and they report feeling confused, angry and “dumped” after physician consult.6
Such uncertainty about FNSD suggests that its etiology may not be easily explained by physicians or comprehensible to patients. Traditionally, the etiology of FNSD has been explained in the context of psychoanalytic theory as a physical manifestation of psychological distress, and many physicians continue to use this as a simple explanation in clinical settings. However, there is little supporting empirical evidence for this hypothesis,9 and patients have been found to respond negatively to psychiatric explanations for physical symptoms.10 There is evidence that rates of trauma, stress and psychiatric comorbidities are higher in patients with FNSD, but recent research has demonstrated low incidence of physical or psychiatric diagnoses to directly explain patients’ symptoms,11,12 and trauma is present in only about one-third of patients.13 No single causal mechanism has been found; instead, predisposing factors vary among individual patients. As a result, the DSM-5 diagnostic criteria for FNSD have removed preceding stressors as a requirement, instead focusing on positive symptoms.1 Several cognitive and neurobiological etiological models have been proposed for medically unexplained illness and FNSD symptoms.14–19 Given recent research and the shift in diagnostic criteria, we provide a review of recent research on the predisposing and reinforcing factors for FNSD. Then, integrating information from other models, we present an integrated etiological summary model of FNSD.
Predisposing factors
Research has demonstrated heterogeneity in the vulnerabilities for FNSD, and individual patients may not experience the same combination of predisposing factors (Table 1). Below is a review of factors that may predispose patients to FNSD.
Overview of FNSD predisposing factors
Trauma/psychiatric symptoms
Trauma and psychiatric symptoms have long been regarded as the cause of FNSD, but research findings in this area have been inconsistent. Patients with FNSD have increased general trauma history,20 and a recent meta-analysis found that 33% of patients with PNES had a history of childhood sexual abuse.21 However, the meta-analysis concluded that there was not enough evidence to establish a causal relationship between childhood sexual abuse and PNES. Still, there is a demonstrated link between PNES and trauma,21 suggesting that trauma is a predisposing factor for the development of FNSD. As well, the magnitude of trauma experience is related to the severity of FNSD symptoms.22,23 This finding has been supported by a recent study demonstrating that childhood abuse burden was associated with left anterior insular volume reductions in women with FNSD.24
Findings related to the association between FNSD, stressors and psychiatric conditions have also been inconsistent. About one-third of patients with FNSD have normal scores on psychological measures, similar to patients with organic movement disorders.25 Further, 2 recent studies found no difference in reported stressors between patients with FNSD and controls. In a group of pediatric patients, all denied history of sexual abuse or trauma, and 25% denied even mundane stressors.26 A study of adults found no difference in stressful events between patients with PNES, patients with epilepsy or controls, but patients with PNES self-reported greater stress and demonstrated fewer coping skills.27 This result was consistent with a study that found no difference in the number or impact of stressful life events between patients with FNSD and controls, but did find that both cortisol (hypothalamic–pituitary–adrenal axis) and α-amylase (adrenergic axis) levels were higher in patients with FNSD. Patients with FNSD and controls responded to a social stress test with similar increases in cortisol and α-amylase, but patients with FNSD self-reported significantly greater stress, which correlated with α-amylase levels.28 This finding suggests that patients with FNSD may perceive stress differently and have fewer skills to cope with stress.
Although some studies have found no significant increase in comorbidities, such as depression, anxiety or personality disorders,25,29 others have found increased prevalence of psychiatric disorders in patients with FNSD. Many patients with PNES have reported panic symptoms before PNES onset, 30 but evidence about anxiety comorbidity is mixed. Some studies have demonstrated high anxiety in patients with PNES,31 but others have found no relationship.32 However, studies found that no anxiety could be the result of a lack of anxiety awareness: some patients with PNES have elevated physiologic arousal but deny anxiety.33 Additionally, some patients with FNSD have reported greater alexithymia (inability to identify and describe their emotions),34 as well as elevated scores on the hypochondriasis and hysteria scales and lower scores on the depression scale of the Minnesota Multiphasic Personality Inventory-2.35 Dissociative disorders are also common psychiatric comorbidities in this population, and the presence of a comorbid dissociative disorder is associated with more severe psychopathology in patients with FNSD.36
While the evidence is not strong enough to indicate direct causality, there is an established connection between FNSD, trauma and psychiatric symptoms, suggesting that these factors, in combination with other predisposing factors, can increase the risk of developing FNSD.
Somatic symptoms
Many patients with FNSD have experienced other medically unexplained symptoms in addition to their functional neurological symptoms.37 Between 57% and 82% of patients with PNES have a history of other medically unexplained symptoms31,37 and have rated their general health as worse than patients with epilepsy.38 Several explanations have been proposed for this increased experience of somatic symptoms. Some research suggests that increased somatization in patients with FNSD may be the result of heightened awareness of physical symptoms. Impairment in sensorimotor gating has been associated with FNSD, suggesting difficulty integrating information from internal and external environments.39 However, other studies have suggested that increased somatization in patients with FNSD could be because of somatosensory amplification — the interpretation of somatic symptoms as injurious, extreme and distressing.40 As well, parental reinforcement of children’s illness behaviour is associated with those children’s concept of their illness, often resulting in beliefs and symptoms incongruent with their actual state of health and persisting into adulthood.41
Illness exposure
Patients with FNSD frequently experience a precipitating physical event before the onset of FNSD.12 Peripheral injury was found in the majority of patients with functional dystonia, 42 while 20% of patients with functional weakness had experienced physical injury to the affected limb near symptom onset.43 This link has been consistently reported since 1965, suggesting that physical trauma may play a significant role in FNSD onset.44
Additionally, many patients with FNSD have a comorbid neurological disorder. Epilepsy prevalence in patients with PNES has been reported to be from 4% to 58%.45 One-third of patients with FMD were reported to have a significant neurological history,12 and 25% had a comorbid organic movement disorder.46 People with PNES and FMD are also more likely to have structural or functional brain abnormalities.47,48
In addition to personal illness experiences, patients with FNSD have often been exposed to others with illness. Medically unexplained symptoms in adulthood have been associated with prior experience of family illness,49 and with professions in the medical field.11 One study reported that 66% of patients with PNES had witnessed an epileptic seizure before PNES onset,50 and more than one-third had a family history of epilepsy.51 News media, television and movies are other common sources of exposure to diseases, and media coverage of a disorder has been associated with increased presentation to physicians with concerns about the disorder.52 These personal and peripheral experiences of illness help shape beliefs about physical symptoms and health and may lead to symptom monitoring.49
Symptom monitoring
Compared with patients with anxiety, patients with FNSD have demonstrated significant impairment in habituation to tones, which was interpreted as a deficit in selective attention.53 Another study found that patients with FMD were less likely to accurately report their heartbeat than controls, instead focusing on external body features.54 As well, fMRI research has shown increased self-monitoring in patients with lateralized paresis of the arm.55 Furthermore, when attention is distracted from the affected area, FMD symptoms decrease and sometimes subside.56
Neurobiological factors
Three processes have been implicated in the neurobiology of FNSD: abnormal attentional focus on the affected area, beliefs and expectations about illness, and deficits in sense of control over one’s actions.57 Research has shown deficits in patients with FMD in movement that they had explicit, conscious control of, but no difference in performance of tasks that relied on automatic factors, suggesting that explicit movement may allow for increased attention on the production of movement in FMD.58
Beliefs or expectations about health can also influence functional symptoms. Patients with FMD request less information than healthy controls before they form a decision, and they change their decision more frequently when presented with new contradictory evidence. This “jumping to conclusions” bias could be a risk factor for inappropriate updating of active inference, the theory in which the brain predicts and explains sensory input through past experiences.57 Additionally, patients with functional tremors self-reported tremor occurrence for 80% to 90% of their waking day, but objective measurement indicated that they had an average of only about 30 minutes of tremor per day. This overestimation was significantly greater than that in patients with organic tremor, suggesting that top–down prediction of constant tremor may prevent perception of time without tremor in patients with FMD.57 Research has also demonstrated the power of symptom expectation, showing that those who expected to experience analgesia in parts of their body reported analgesia in exactly those areas.59 This finding has been incorporated into several etiological models for general medically unexplained physical symptoms and FNSD.14–17
Patients with FMD tend to have a decreased sense of agency or control over their actions. One study compared brain activity in mimicked tremors and functional tremors in patients with FNSD; it found hypoactivity in the right temporoparietal junction and lower functional connectivity between the right temporoparietal junction, sensorimotor cortices and limbic regions during functional tremors, suggesting that symptoms are perceived to be involuntary despite the use of voluntary motor pathways.60,61 These findings of decreased functional connectivity between the sensorimotor cortices and the temporoparietal junction were later replicated with a larger sample size of patients with FMD.62 A computerized task has also been used to assess sense of agency in FMD by measuring patients’ action–effect binding. Compared with controls, patients with FMD showed increased perceived time between their actions and an effect, suggesting a decreased sense of control over their actions.63
Several functional and structural abnormalities have consistently been present in patients with FNSD, especially in motor-processing regions and regions with dual motor- and emotion-processing functions. Compared with matched controls, patients with FNSD showed increased activity in the amygdala,61,64,65 supplementary motor area60,65 and periaqueductal grey matter (associated with the freeze response of fear)61 in response to negative emotions across several studies.61,64,65 Increased connectivity was demonstrated between the right amygdala and the right supplementary motor area when participants were presented with fearful and happy faces64 and in response to recall of stressful life events.65 This finding provides a potential mechanism by which certain stressors are associated with functional symptoms. Because of observed neural impairments in areas of the brain associated with emotional, perceptual and intentional awareness, Perez and colleagues66 suggested that patients with FNSD might experience a “neural functional unawareness,” which could also help conceptualize the brain–behaviour relationship in this disorder.
There are also some emerging functional and structural neuroimaging findings. Research has found abnormal functional connections in areas associated with cognitive control, behavioural inhibition and perceptual awareness.66 In patients with FNSD in response recall of stressful life events, enhanced activity has been found in the left dorsolateral prefrontal cortex, right supplementary motor area and temporoparietal junction, and decreased activity in the left hippocampus.65 Evidence also suggests abnormal brain activity in areas regulating sensory integration (posterior parietal cortex and angular gyrus regions).66
In terms of structural abnormalities, 1 study found no difference in insular volumes between patients with FNSD and controls. However, patients with FNSD who had self-reported severely impaired physical health had reduced left anterior insular grey matter volumes, and patients with FNSD participants who had self-reported severely impaired mental health had greater volumes of posterior-lateral cerebellar grey matter than controls.67 Two studies have demonstrated decreased grey-matter volumes in the thalamus and basal ganglia in patients with FNSD.48,68 Further, Labate and colleagues69 found abnormal cortical atrophy in the right motor and premotor areas and the right and left cerebellum in patients with PNES. Structural abnormalities have also been found in children and adolescents with FNSD, demonstrating greater volume in the left supplementary motor area, right superior temporal gyrus and dorsomedial prefrontal cortex.70
It is important to note that because these findings rely on cross-sectional designs, it is unclear whether these structural and functional abnormalities are the cause of functional symptoms or a consequence of FNSD. However, some data suggest that these findings are the result of experience-dependent neuroplasticity of the brain, or the brain’s ability to change in response to the environment or learning.71 Labate and colleagues69 demonstrated that higher depression scores were associated with decreased grey matter in the premotor regions, and Aybek and colleagues72 found a trend for an association between greater grey matter in supplementary motor regions and duration of FNSD, and for an association between increased grey matter in the left premotor cortex and symptom severity. Additionally, Aybek and colleagues28 demonstrated that higher sexual abuse rates were associated with a weakened objective response to stress in patients with FNSD. The experience-dependent neuroplasticity explanation is also consistent with research in children, which found that greater supplementary motor area volumes were associated with faster emotion-identification reaction time.70 Unlike in adults, no differences have been displayed in the basal ganglia, thalamus or cerebellum of children and adolescents with FNSD,70 suggesting that decreases in grey matter in these areas could be due to the duration of FNSD symptoms.70 However, additional longitudinal neuroimaging data are needed to determine which effects are the result of an experience-dependent neuroplasticity reaction to FNSD symptoms; an experience-dependent neuroplasticity response to adverse life events; and/or a genetic predisposition to reacting to stress with functional neurological symptoms.
Reinforcing factors
In addition to predisposing factors, two other factors may reinforce FNSD (Table 2).
Overview of FNSD reinforcing factors
Sick role
The sick role is the acceptance of illness by the patient, and it is governed by certain social expectations, including not being responsible for one’s condition and exemption from normal social responsibilities.73 As noted above, expectations and beliefs about illness have been found to influence FNSD symptoms. Therefore, expectations associated with the sick role may increase FNSD symptoms.
Many studies have found evidence of the sick role in patients with FNSD. One found that only 20% of patients with PNES were employed by the time of referral for electroencephalography. Receipt of health benefits significantly increases after PNES diagnosis, and patients with PNES are more likely to receive benefits than patients with epilepsy.6 Research has found that patients with FNSD who worked were more than 5 times more likely to become symptom-free.74 Another study found that patients adopted the sick role as an important part of their identity,75 and patients with FNSD avoided normal social interactions.76 However, some studies have found contradictory evidence about the sick role in patients with FNSD. One found a decrease in general health care utilization,77 and another found that health care costs decreased 12 months after diagnosis.78 While the sick role may not be present for all patients with FNSD, it may reinforce symptoms in some.
Secondary gain
Once FNSD has developed, patients may experience secondary gain, an intrinsic or extrinsic benefit that reinforces and maintains FNSD. Traditionally, secondary gain has been described as an etiological factor for FNSD from a psychodynamic perspective, serving as an unconscious attempt to escape unwanted psychological distress.79 The concept of secondary gain as a causal mechanism is contradicted by the absence of stressors before the onset of FNSD in many patients, 26,27 but there is evidence that it may reinforce symptoms or provide a disincentive for symptom resolution in some patients.74 It has been suggested that PNES are maintained by operant conditioning through both positive and negative reinforcement, such as the release from stress associated with employment or increased attention from family or friends,80,81 or the receipt of disability benefits.82
Proposed etiological models
Although traditional etiological understanding of FNSD relied simply on the psychodynamic explanation of a physical manifestation of psychological distress as the cause of the disorder, recent etiological models have acknowledged the heterogeneity of patients with FNSD. Several cognitive and neurobiological etiological models have been proposed for medically unexplained symptoms, PNES and FNSD.
Brown and Reuber recently proposed a model that provides an integrated behavioural and psychological etiological explanation.15,16 This model is based on ideas from Brown’s cognitive model of unexplained illness, in which misinterpretation of physical symptoms is affected by “rogue representations,” or information in the cognitive system about the cause of physical symptoms, which can be attained through personal experience, the observation of others’ experiences or sociocultural influence about health.14 Similarly, in their cognitive conceptual model for PNES, Brown and Reuber described the “seizure scaffold” as the central feature of PNES. The seizure scaffold is described as automatic activation of seizure behaviour from memory, occurring during autonomic arousal as a result of threat-processing.15,16
Voon and colleagues18 have proposed a neurobiological model in which FNSD occurs because of a combination of increased emotional arousal in the amygdala at symptom onset and a “previously mapped conversion motor representation,” possibly as a result of a prior physical precipitating event. They suggest that the “previously mapped conversion motor representation” is triggered and cannot be inhibited18 due to abnormal functional connectivity between the limbic structures and the supplementary motor area60 and higher activity in the right amygdala, left anterior insula and bilateral posterior cingulate.64
In another neurobiological model for FNSD, Edwards and colleagues17 have proposed a Bayesian account for FNSD. They suggest that functional symptoms are the result of actions based on inferences. These inferences are mediated by expectations about symptoms, past emotional and illness experiences and body-focused attention. Functional symptoms are the result of failures of inference occurring outside of conscious control.17
Based on their work with children and adolescents, Kozlowska and colleagues83 hypothesized a model of PNES based on Janet’s dissociation model.84 Their model suggests that a range of dissociative brain processes become triggered in response to cortical arousal, resulting in abnormalities in brain function and connectivity. Cortical arousal can be the result of illness, injury, emotional distress or trauma. This model proposes that in response to cortical arousal, the brain shifts into a defensive state in which behaviour becomes reflexive rather than voluntary. The authors suggest that FNSD is the result of this defensive state, in which the basal ganglia, midbrain and brain engage in reflexive behaviours.83
Integrated etiological summary model
The etiology of FNSD is complex and often results from a combination of factors that vary by the person. Patients and health care providers often report frustration and confusion about FNSD, which can impede accurate and timely diagnosis and hinder development of effective treatments. To integrate the recent research and current cognitive and neurobiological etiological models reviewed above, and to summarize the findings in a way that is easily comprehensible to patients, we describe the integrated etiological summary model for FNSD, which allows for the heterogeneity observed among patients and proposes a causal mechanism.
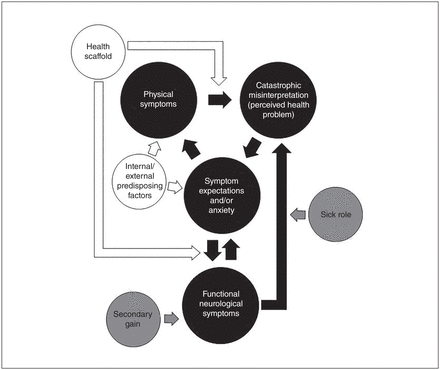
Figure 1 illustrates the sequence of events that is suggested to occur in the establishment and maintenance of FNSD.
The integrated biopsychosocial model for functional neurological symptom disorder. White represents predisposing factors, black represents the main model pathway and grey represents reinforcing factors.
Internal/external predisposing factors
Because of heterogeneity among patients with FNSD, it is likely that many combinations of predisposing factors yield similar functional symptoms. Some patients with FNSD may have a history of trauma or be predisposed to have anxiety and/or increased physical symptoms.31,37–39,41,50 Others may experience increased physiologic arousal without subjective reports of anxiety,33 but some may have no history of anxiety or psychiatric comorbidities. Many patients with FNSD experience functional symptoms after a physical injury or other neurological disease.12,42–46 As suggested by Kozlowska and colleagues,83 these factors may cause experience-dependent neuroplastic structural and functional changes in the brain, or epigenetic changes that may increase the risk of developing FNSD.
Model pathway
The onset of FNSD may be gradual or sudden. In patients with gradual onset, symptom presentation and duration are progressive, worsening over time. As proposed in Clark’s cognitive model of panic,85 anxiety leads to physical symptoms. However, some patients with FNSD do not report anxiety about their symptoms. Instead, they may be predisposed to increased awareness or greater experiences of physical symptoms; these symptoms are then misinterpreted as a health problem. Misinterpretation of physical symptoms is influenced by a learned mental representation of physical symptoms created by a series of beliefs, expectations and motor activities formed through cultural beliefs, past injury, illness, experience and/or personal knowledge.14–17 These cognitive representations are referred to by Voon and colleagues18 as “previously mapped conversion motor representations,” by Brown14 as “rogue representations” for medically unexplained symptoms, and by Brown and Reuber15 as the “seizure scaffold” for PNES. As noted above, illness beliefs and expectations held by patients with FNSD may not be limited to exposure to neurological conditions or symptoms. Because this model is focused on the production of all functional neurological symptoms, it uses the broader term “health scaffold,” which encompasses all illness experiences, including personal illness, parental anxiety about the patient’s health as a child, illness of family or friends, job in a health profession, witnessed event of a stranger in public and cultural beliefs that certain symptoms are associated with a particular condition (e.g., forgetting one’s name is associated with dementia).14,41,49,50,52 Additionally, through news coverage of medical conditions and illnesses portrayed on television and movies, there is ample opportunity for illness exposure and health scaffold development. Intensified by the “jumping to conclusions” bias in some patients,17 a strong health scaffold increases sensitivity to even minor physical symptoms and reinforces beliefs that physical symptoms signify serious illness. Misinterpretation of symptoms as an illness then leads back to expectation of symptoms and/or anxiety, which produces additional physical symptoms and continues the cycle, further generating symptoms until it results in a symptom consistent with FNSD. In instances of sudden onset, the occurrence of symptoms may not cycle through the pathway as in gradual onset. Patients may have a significant trauma or injury, such as a car accident, and the associated physical symptoms are misinterpreted, resulting in the expectation and immediate onset of symptoms consistent with FNSD.
Mechanism of action
We propose the mechanism by which FNSD is produced can be explained in the context of the placebo effect, which is the result of a combination of classical conditioning and explicit expectancies.86 Ivan Pavlov, who first discovered classical conditioning, was the first to propose it as a causal mechanism for FNSD.87 Classical conditioning occurs when an unconditioned stimulus is paired with a neutral stimulus until the neutral stimulus (then called the conditioned stimulus) elicits the reflexive unconditioned response in the absence of the unconditioned stimulus (then called the conditioned response). This is consistent with the high rate of precipitating physical events and the common co-occurrence of epilepsy and PNES. Classical conditioning may occur through repeated pairing of the unconditioned stimulus and the neutral stimulus or, if the event is sufficiently significant, through a single pairing. In a recent book chapter, Carson and colleagues suggested that the manifestation of FNSD may occur through single event of classical conditioning and be mediated by panic as the conditioned response.88 This suggestion is supported by evidence that many PNES first present as fainting in a social situation; the fainting causes anxiety, and subsequent occurrences are believed to be triggered by small fluctuations in emotion or neutral stimuli or mediated by panic.88,89 Multiple-exposure classical conditioning could explain the occurrence of FNSD in some patients, such as those with a personal or family history of neurological disorders. If the patient has repeatedly experienced seizures or been exposed to a family member’s seizures, neutral stimuli present during each of the experiences may produce a seizure-like conditioned response.
In addition to classical conditioning, negative symptom expectations have been found to directly modulate several neurochemical systems, resulting in increased symptoms.90 Expectation of a relationship between 2 stimuli can also result in classical conditioning without prior pairing of the stimuli. Dawson and Grings91 discovered that verbal information about a relationship between the unconditioned stimulus and neutral stimulus was sufficient to produce a conditioned response, consistent with high rates of personal illness experience and observed exposure to illness through family members or the media in patients with FNSD. Therefore, the health scaffold can contain classically conditioned neurological behavioural responses to neutral (conditioned) stimuli.
When a person experiences a normal physical symptom associated with their health scaffold, they misinterpret the symptom as a medical condition, and their expectation of symptoms and/or anxiety increases. If the symptom has been classically conditioned to a neurological behaviour in their health scaffold, it results in reflexive functional neurological behaviour. For example, someone with a history of epilepsy may have experienced headache (neutral stimulus) before their seizures (unconditioned response), so that headaches become a conditioned stimulus to seizure behaviour (conditioned response) and further develop their health scaffold. When the symptoms are experienced outside the context of an epileptic seizure, they are misinterpreted as an epileptic seizure, resulting in an expectation of symptoms, and functional seizure behaviour is automatically triggered.
While this mechanism explains the sudden onset of symptoms, many patients with FNSD experience a gradual onset. This can be explained through shaping, or the differential reinforcement of successive approximations.92 A physical symptom may trigger the health scaffold, resulting in the misinterpretation of the symptom as a health problem and leading to the expectation of symptoms and/or an increase in anxiety, but the symptom may not be conditioned to elicit a reflexive neurological response. However, expectation of symptoms has been found to increase reported experience of symptoms.59 Once the symptom recurs, the belief that the symptoms are due to a health problem is reinforced, which again results in expectation of symptoms and/or anxiety. As this cycle continues, most often on a preconscious level, symptoms are gradually shaped to become more frequent and prominent, producing additional symptoms. Once this results in a symptom that has been conditioned to a neurological response, functional neurological symptoms are triggered. This process of shaping may also be responsible for the gradual progression of functional symptoms and frequent co-occurrence of multiple functional symptoms.
Once FNSD symptoms are produced, new physical symptoms and situations occurring just before or simultaneously with the functional symptoms can be conditioned to trigger FNSD, consistent with Clark’s cognitive model of panic85 and Carson and colleagues’ suggestion that panic may mediate conditioning as the conditioned response.88 For example, heart rate increases during FNSD symptoms because of increased anxiety/panic associated with an episode can lead to FNSD symptoms (conditioned response) being triggered by an increase in heart rate (conditioned stimulus) when angry or while running.
This model is consistent with the Bayesian account of FNSD, in which it is posited that functional symptoms are the result of actions (conditioned response) based on failures of inference (misinterpretation of symptoms) from beliefs founded on prior experiences (health scaffold) and sensory evidence (physical symptoms).17
Reinforcing factors
After the onset of FNSD, the sick role and secondary gain reinforce and maintain FNSD symptoms. Reinforcers of the sick role include staying home, abstaining from responsibilities, family members acting as caregivers and repeatedly going to the emergency department.
Secondary gain reinforces symptoms through operant conditioning93 by following the symptoms with a rewarding response. This includes increased attention from family and friends (positive reinforcement) and decreased aversive responsibilities, such as attending work (negative reinforcement). The sick role and secondary gain overlap, but are reinforcing in different ways. For example, staying home from work reinforces a person’s belief that they are sick by providing cognitive support for the expectation that they are exempt from normal social responsibilities and also negatively reinforces symptoms by removing work stress.
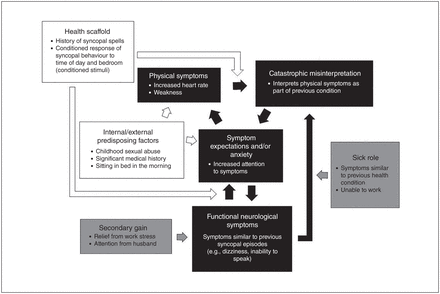
Reinforcing factors maintain episodic functional symptoms but also contribute significantly to symptoms occurring constantly, such as anesthesia or paralysis. After the initial onset of symptoms, expectation of continued symptoms, acceptance of the sick role and secondary gain work in combination to maintain FNSD. For example, once paralysis begins, the patient develops an expectation of being paralyzed every day. Upon wakening, they expect paralysis to continue, and symptoms are maintained. This is reinforced by staying home from work and receiving help from family members, which is rewarding. Figure 2 uses a real-life case example to demonstrate the clinical application of the integrated etiological summary model.
Integrated biopsychosocial model, case example. A 23-year-old woman with history of sinus node dysfunction, syncopal spells from documented sinus arrest and a history of childhood sexual abuse, for which she has previously sought psychological treatment. She had a pacemaker implanted to treat her medical diagnosis, which resolved her syncopal episodes. However, new episodes began the day after the pacemaker implantation, with symptoms similar to those she had experienced before, such as dizziness, but without full loss of consciousness. Her episodes occurred at the same time of day and in the same location as before. She was referred for neurological assessment, which demonstrated normal sinus rhythms and no EEG changes during the events, and she was diagnosed with PNES. White represents predisposing factors, black represents the main model pathway and grey represents reinforcing factors. EEG = electroencephalography; PNES = psychogenic nonepileptic seizures.
Extinction and relapse
Finally, this model also accounts for common relapse of functional neurological symptoms after remission.94 When a conditioned response is extinguished, the response is not unlearned. Instead, new learning occurs that is stored with previous learning, resulting in 2 responses for the same stimulus, and the resulting response is determined by the context of the situation, such as environment, mood or time. Therefore, the extinguished behaviour can relapse given a certain context.93
Supporting neurobiological evidence
In support of this model, the findings of several neuroimaging studies are consistent with the concept of placebo effect and classical conditioning. The automatic reflexive response produced through classical conditioning is consistent with the decreased sense of agency for symptoms. Because of abnormal functional connectivity found between the limbic structures and motor areas60 and higher activity in the amygdala, insula and cingulate,64 the “previously mapped conversion motor representation” is triggered and cannot be inhibited (conditioned response within the health scaffold).18 It is also interesting to note that the same areas of the brain with increased activation in this model are associated with classical conditioning.95 Several other studies also demonstrate similar structural and functional patterns between classical conditioning and FNSD. For example, increased stress has been found to increase the acquisition and consolidation of classically conditioned responses in animals and humans. Specifically, noradrenaline release associated with stress facilitates the acquisition of fear conditioning, while glucocorticoids facilitate the consolidation of classical conditioning.96 This may explain why there are high rates of patients with FNSD and a history of stress and trauma. As well, greater cerebellar volume is associated with higher levels of classical conditioning,97 which could explain the association demonstrated by Perez and colleagues67 between greater self-reported severely impaired mental health and greater volumes of posterior-lateral cerebellar grey matter in patients with FNSD. Research assessing pain-related classical conditioning has demonstrated that anticipation of a classically conditioned response and placebo effects due to expectations after verbal suggestion result in increased activation in areas of the brain related to attentional (posterior cingulate, anterior cingulate) and emotional (amygdala, hippocampus) processing, 98 similar to the increased activation found in patients with FNSD.18,99 Specifically, increased amygdala activity has been consistently demonstrated in FNSD,61,64,65 and the amygdala is the gate for the physiologic expression of classically conditioned behaviour.96
Conclusion
The etiology and maintenance of FNSD result from a variety of precipitating and reinforcing factors, and insufficient etiological understanding by physicians and patients impedes diagnosis and treatment. Although no model may be able to capture the etiological pathway for all patients with FNSD, our summary model presented here integrates current research into FNSD from various fields and recent etiological models. We have also proposed the placebo effect as the mechanism of action and emphasized how much of the research on the placebo effect and classical conditioning is consistent with the neurobiological evidence for the production and maintenance of FNSD. While others have discussed classical conditioning as a causal mechanism,87,88 this paper expands the explanation of research on the placebo effect and classical conditioning to account for additional aspects of FNSD, including its consistency with patients’ decreased sense of agency and other structural and functional neuroimaging findings; the shaping of symptoms over time; episodic and continuous symptoms; positive symptoms (tremors) and negative symptoms (paralysis); and the high rate of relapse of symptoms over time (extinction and spontaneous recovery).93
This model poses multiple testable research hypotheses. The overlap between the neurobiological evidence for FNSD and classical conditioning provides ample opportunity for additional research. There are also many evidence-based interventions that address etiological factors related to classical conditioning, such as exposure and response prevention for anxiety, which will provide a foundation to inform treatment development for FNSD. Future studies can assess the overlap between brain function during classical conditioning and FNSD, measure whether symptoms are shaped to evolve over time, and evaluate patients’ acceptance of the diagnosis based on this explanation. Research can also examine whether FNSD symptoms are altered by treatment that restores a sense of agency over symptoms or by using extinction techniques such as exposure with response prevention.
This summary model explains symptoms as automatic conditioned reflexes to certain stimuli, and psychiatric factors are noted as potentially influential but unnecessary for the development and maintenance of FNSD. Classical conditioning and the placebo effect are commonly understood concepts that do not have psychiatric connotations. Since patients often reject FNSD diagnosis and prefer a medical diagnosis over a psychiatric diagnosis,10 this model could provide physicians with an explanation that is more acceptable to patients, potentially increasing patients’ willingness to pursue treatment.
Acknowledgments
A. Fobian is funded by award number 5K23DK106570 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, National Institute of Diabetes and Digestive and Kidney Diseases, or the National Institutes of Health.
Footnotes
Competing interests: None declared.
Contributors: A. Fobian conducted a literature review, integrated previous research, and outlined and described the summary model. L. Elliott also also conducted the literature review. Both authors wrote and reviewed the article, approved the final version for publication, and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received September 22, 2017.
- Revision received February 23, 2018.
- Accepted March 26, 2018.