Abstract
Background: To date, antidepressant drugs show limited efficacy, leaving a large number of patients experiencing severe and persistent symptoms of major depression. Previous open-label clinical trials have reported significant sustained improvements with deep brain stimulation (DBS) of the subcallosal cingulate gyrus (SCG) in patients with severe, chronic treatment-resistant depression (TRD). This study aimed to confirm the efficacy and measure the impact of discontinuation of the electrical stimulation.
Methods: We conducted a 6-month double-blind, randomized, sham-controlled crossover study in implanted patients with previous severe TRD who experienced full remission after chronic stimulation. After more than 3 months of stable remission, patients were randomly assigned to 2 treatment arms: the ON–OFF arm, which involved active electrode stimulation for 3 months followed by sham stimulation for 3 months, and the OFF–ON arm, which involved sham stimulation for 3 months followed by active stimulation for 3 months. The primary outcome measure was the difference in the 17-item Hamilton Rating Scale for Depression (HAMD-17) total score between sham and active stimulation.
Results: We enrolled 5 patients in our trial. A Friedman repeated-measures analysis of variance revealed a significant effect of treatment (χ21 = 5.0, p = 0.025) in patients with higher depression scores during sham stimulation. At the end of active stimulation, depression was remitted in 4 of 5 patients and none of them had experienced a relapse, whereas at the end of sham stimulation, 2 patients remained in remission, 2 relapsed and 1 showed a progressive worsening without reaching relapse criteria.
Limitations The small sample size limited the statistical power and external validity.
Conclusion: These preliminary findings indicate that DBS of the SCG is an effective and safe treatment for severe forms of TRD and that continuous electrical stimulation is required to maintain therapeutic effects.
Introduction
Major depression is among the most prevalent and costly disorders and is the third leading cause of disease burden worldwide.1 Unfortunately, pharmacotherapy is efficacious in only two-thirds of patients, even after 4 trials with different drugs,2 leaving a substantial percentage of patients with transient, partial or no responses. Actually, treatment-resistant depression (TRD) has an estimated prevalence of 1%–3%,3 which has urged the development of new strategies to overcome the limitations of existing therapeutic approaches (including pharmacotherapy and psychotherapy). Among these novel strategies, deep brain stimulation (DBS) has been successfully used in patients with TRD.
Deep brain stimulation is a stereotactic functional neurosurgery technique that consists of the implantation of electrodes connected to a neurostimulator in different brain areas related to the given disease. New applications of this technique are being explored in some patients with treatment-resistant psychiatric illnesses, such as treatment-resistant obsessive–compulsive and major depressive disorders.3
The use of DBS for TRD has been attempted in different brain targets, showing promising and encouraging results.4–8 To date, the findings support the efficacy of the technique in the subcallosal cingulate gyrus (SCG),6,7,9 the nucleus accumbens10,11 and the ventral capsule/ventral striatum.12 Although these previous studies express agreement with even the long-term safety and antidepressant efficacy of DBS in TRD, data have been obtained using uncontrolled or open-label designs.5 Given the complexity of the procedure, it is still unclear whether the antidepressant response to DBS can be fully attributed to electrode stimulation, to local changes due to the implantation of metal electrodes with eventual alterations of network function or, less likely, to placebo effects. Hence, controlled studies with experimental validity are needed to clarify the role of the different interventions in the antidepressant response to DBS.
Here we report on, to our knowledge, the first double-blind, randomized, sham-controlled crossover study conducted to confirm the efficacy and measure the effect of discontinuation of electrical stimulation of the SCG in a group of patients with TRD who achieved full remission after continuous DBS.
Methods:
Patients
A sample of depressed patients with a severe and highly resistant form of major depression, refractory to multiple antidepressant drug trials, psychotherapy or electroconvulsive therapy, was selected for DBS of the SCG (details of the surgical procedure and sample have been published previously7). After discharge from the Neurosurgery ward, patients remained in the Psychiatry ward for 2 days and were then followed by their psychiatrist (R.P.E.) in the outpatient clinic.
Patient recruitment occurred between February 2008 and December 2009. To be included in the present trial, implanted patients had to achieve stable clinical remission with continuous stimulation. Stable clinical remission was defined a 17-item Hamilton Rating Scale for Depression (HAMD-17) score below the cut-off of 8, maintained for at least 3 months.
The trial was conducted in the Department of Psychiatry of the Hospital de la Santa Creu i Sant Pau in Barcelona, Spain, in accordance with the Declaration of Helsinki. The ethics committee of the hospital approved our study protocol, and all patients gave written informed consent. The clinical trial was also approved by the Agencia Española de Medicamentos y Productos Sanitarios (Spanish regulatory drug agency). The protocol is registered at ClinicalTrials.gov with the identifier NCT01268137.
Study design
Our study was a randomized, double-blind, controlled crossover clinical trial with a total duration of 6 months comprising 2 consecutive 3-month phases. After a stable clinical response to DBS, patients were randomly assigned to 1 of 2 arms: the OFF–ON arm, which involved sham stimulation for 3 months followed by active stimulation for 3 months, or the ON–OFF arm, which involved active stimulation for 3 months followed by sham stimulation for 3 months. We generated the random allocation sequence using the pseudorandom numbers generator in SPSS software version 18. Given the small number of patients, no blocking was used. Random assignment was done with sealed envelope selection to conceal the sequence from the rest of the researchers and to ensure that only the investigator who manipulated the neurostimulator (A.G.) knew the allocation pertaining to each individual. This investigator did not carry out any assessment or any other intervention during the trial period. Given the small number of patients who could be included and randomly assigned, we performed no power calculations to determine sample size.
For each individual, the following stimulation parameters were maintained during the active stimulation periods of the trial and after the trial: frequency 130–135 Hz, amplitude 3.5–5 V and pulse width 120–240 ms. Although pulse widths were higher in our group, most values were similar to those used in previous studies of SCG DBS for depression.4,6,9 In the case of 2 visits in which HAMD-17 scores above 14 were recorded, patients were withdrawn, the stimulator was turned on (for patients in the sham stimulation phase), or the stimulation parameters were adjusted (for patients in the active stimulation phase). Clinical assessments were performed every 2 weeks by a single psychiatrist (R.P.E.), who was unaware of the stimulation assignment. Concurrent antidepressant medication was held constant during the trial, and it was adjusted at the end of the trial, if necessary.
Outcome measures and data analyses
The primary outcome measures were the HAMD-17 scores in each month of the trial (although patients were visited at least twice a month during this 6-month period). The HAMD-17 scores while on sham stimulation were combined and those while on active stimulation were combined, and then the scores were averaged per treatment to carry out the main analysis. Given the small sample, we used Friedman nonparametric, repeated-measures analysis of variance (ANOVA) to compare active and sham stimulation. We calculated the areas under the curve (AUC) of HAMD-17 scores for active and sham stimulation during the 2 phases of the trial using a trapezoid method, which is a common approximation of an area under a set of adjoining straight line segments. Missing values were replaced using the last observation carried forward method. We considered results to be significant at p < 0.05.
Rates of remission and relapse were also calculated. Remission was defined as a HAMD-17 score lower than 8, and relapse was defined as a worsening of depressive symptoms with a HAMD-17 score higher than 14.
Results:
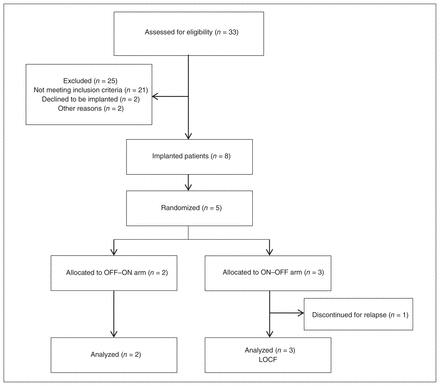
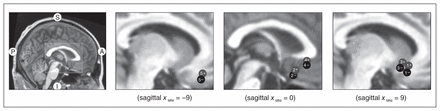
In December 2009, 8 patients had received implants for DBS (6 women and 2 men). Figure 1 shows the study selection process and final sample. Five of 8 patients achieved stable clinical remission with DBS of the SCG and were then enrolled and completed the present trial. Two patients were allocated to the OFF–ON arm, and 3 to the ON–OFF arm. One patient (patient 4, ON–OFF arm) was withdrawn from the trial during the OFF phase because of a serious relapse. Another patient (patient 1, OFF–ON arm) also experienced a severe relapse by the end of the OFF phase, but there was no need to withdraw this patient. Sample characteristics are shown in Table 1. The severity of clinical symptoms and the demographic characteristics at trial initiation did not differ between the 2 treatment arms (all p > 0.21). Table 2 provides information on patient allocation, stimulating parameters and active electrodes at the beginning of the trial as well as pharmacological treatment at trial entry. No antidepressant medication changes were required, which avoided potential confounding factors on DBS efficacy. In addition, Figure 2 displays the location of electrodes in the SCG.
Study selection for the randomized controlled clinical trial. LOCF = last observation carried forward; OFF–ON = 3 mo of sham stimulation followed by 3 mo of active stimulation; ON–OFF = 3 mo of active stimulations followed by 3 mo of sham stimulation.
Location of electrode contacts on a sagittal view of the cingulate gyrus. Circles are schematic representations of electrode contacts (“+” indicates positive contacts and “−” indicates negative contacts). Numbers within circles correspond to each patient. Details of stimulating parameters are given in Table 2.
Clinical and demographic characteristics of the study sample (n = 5)
Patient data on randomization, active contacts and stimulating parameters at the beginning of the trial and the medications prescribed at trial entry
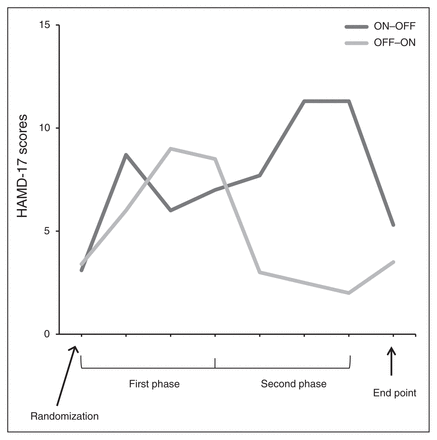
The Friedman repeated-measures ANOVA revealed a significant effect of treatment (χ21 = 5.0, p = 0.025), with higher depression scores during sham stimulation (HAMD-17 scores for each participant averaged over 3 months). The resulting AUC of HAMD-17 scores for patients subjected to active and sham stimulation were 13.9 and 16.3, respectively, during the first phase and 5.0 and 20.8, respectively, during the second phase of the trial (Fig. 3).
Mean change in depression scores based on the 17-item Hamilton Rating Scale for Depression (HAMD-17) for the ON–OFF (3 mo active followed by 3 mo sham) group and the OFF–ON (3 mo sham followed by 3 mo active) group.
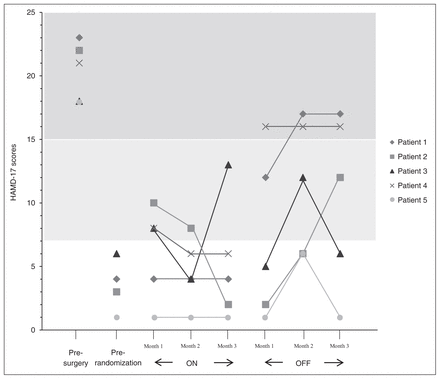
During active stimulation, 4 of 5 patients maintained response scores, and at the end of the active phase none of them had relapsed (4 were remitted patients; Fig. 4). Conversely, during sham stimulation, only 2 patients remained in remission, another 2 relapsed and 1 showed a progressive worsening without reaching relapse criteria.
Individual changes on th 17-item Hamilton Rating Scale for Depression (HAMD-17) scores during active stimulation (ON) and sham stimulation (OFF) periods. Scores on the dark grey background represent relapses; scores on the white background correspond to remissions.
Incidents and adverse events
No other neuropsychiatric complications or general adverse effects (e.g., headache, dizziness, gastrointestinal disturbances, paresthesias) associated with stimulation were identified during the clinical trial period. Adverse events associated with the device or surgery and those that took place before randomization were few and have been described elsewhere.7
Discussion
The results of this first double-blind, randomized, controlled crossover clinical trial indicate that DBS of the SCG may be an effective and safe treatment for patients with severe forms of depression and confirm that continuous electrical stimulation is required to maintain therapeutic effects. The overall clinical impression at the end of the study was that antidepressant response to DBS is lost after cessation of the stimulation, as we observed clear relapses or clinical worsening after discontinuation. Previous clinical trials of DBS of the SCG in patients with TRD were uncontrolled or open label.4–7 Some prior studies reported relapses after accidental DBS cessation (e.g., battery depletion6), and only 1 trial attempted to incorporate a single-blind discontinuation phase, which was interrupted early owing to patient safety concerns.5 Despite growing evidence in support of DBS of the SCG for TRD, a double-blind, sham-controlled study with maximal safety guaranties was strongly needed to dispel doubts about the efficacy of the treatment and to corroborate the importance of maintaining electrical stimulation.
Individuals included in our trial were patients with highly refractory depression who had experienced a sustained remission with chronic DBS of the SCG. The most interesting observation of the present study is that almost all patients maintained remission during the active stimulation phase, and none of them experienced a relapse, whereas 3 of 5 relapsed or worsened with the cessation of stimulation, indicating a direct therapeutic effect of active DBS. Given the blinded and controlled conditions of the trial and the fact that enrolled patients had attained a sustained remission with continuous DBS before entering the trial, our findings do not seem to be due to placebo effects. It is worth mentioning that the duration of the clinical stability before trial enrolment, which ranged from 3 to 9 months (mean 6.2 mo), was long enough that relapses could be considered a consequence of the treatment arms.
The unexpected moderate worsening (mostly owing to anxiety items) during the initial phase of the ON–OFF arm (i.e., active stimulation) may be a random difference due to the small number of patients in each treatment arm. Alternatively, it could also reflect a modest nocebo effect mediated by negative expectations when patients faced the possibility of being randomly assigned to the sham stimulation. This hypothesis seems particularly plausible with these patients, who had achieved complete remission with DBS after years of severe depression. However, symptomatic exacerbation among these patients was more conspicuous when they entered the OFF phase of the trial. The role of negative expectations has been described previously in patients with Parkinson disease who were successfully treated with DBS of the subthalamic nucleus.13,14 Benedetti and colleagues13 showed that motor performance worsened not only when stimulation was turned off, but also when researchers simply pretended to disconnect the device. Whatever the explanation of the initial worsening in our sample, it had a small effect size and was transient, allowing the detection of significant differences between active and sham simulation phases. Despite the group differences during these phases, some patients did not experience any clinical change when the stimulation was turned off. Mayberg and colleagues6 also reported a case in which antidepressant effects were maintained up to 4 weeks after a single-blinded discontinuation of the electrical stimulation. Both observations suggest the existence of long-lasting therapeutic effects of DBS in some depressed patients.
Deep brain stimulation is still unproven and has limited approval by the United States Food and Drug Administration or the European Medicines Agency for treating psychiatric conditions, such as major depression, obsessive–compulsive disorder and Tourette syndrome. In the case of depression, because of heterogeneous patient characteristics and the limited number of studies available — few of them with adequate design — there is a strong need to keep acquiring data to determine the effectiveness/efficacy of the intervention and what brain targets and clinical profiles of patients are the most optimal. A key question, which we addressed in the present study, is whether the active stimulation of electrodes is essential to achieve therapeutic effects. To our knowledge, until now, only 1 previous trial has assessed the efficacy of DBS for TRD using a double-blinded randomized design. The selected target was the ventral capsule/ventral striatum, patients were randomized early after surgery, and the findings were negative (data presented at the 67th Annual Scientific Convention & Program of the Society of Biological Psychiatry15). By contrast, a double-blind crossover design allowed us to test the efficacy of DBS of the SCG in patients who had already responded, providing reliable information on the causal relation of stimulation effects with minimum risks for the patient (i.e., a putative worsening of patients after switching off electrodes was carefully assessed, and, in the occurrence of a relapse, the patient was withdrawn and stimulation adjusted or restarted).
Neuroimaging studies indicate that the SCG plays an important role in the pathophysiology and treatment of major depression.17–19 Indeed, the prefrontal cortex is the higher order association cortex, integrating sensory, limbic and autonomic information.16 It plays a major role in higher brain functions, exerting a top–down control of a large number of cortical and subcortical structures.17 In particular, the SCG cortex is anatomically and functionally related with other cortical, thalamic and limbic areas.18,20,21 Since DBS affects multiple neural structures, such as myelinated axons and, to a lesser extent, cell bodies,22 it is likely that the orthodromic and antidromic axonal stimulation of the SCG may affect neuronal transmission in a large number of brain areas projecting to and receiving inputs from this key area. In agreement with this view, focal and distal effects can be inferred after DBS of the SCG,8 and this could explain why some patients with depression can still maintain their previous improvement in the sham stimulation phase and also why the effects are not immediate when stimulation is activated.
Limitations
Unfortunately, the very small sample size represents a major limitation of the study, so figures should be considered supportive, and statistical analyses are essentially illustrative rather than definitive. Given that only patients in a steady state of remission were randomized, the conclusions should be drawn with some caution owing to possible selection bias. In any case, 5 of 8 patients could be randomly assigned to a trial arm, which is a remarkable proportion of patients for such extremely chronic, treatment-resistant disorders. The findings of prior works by others4–6,9 and by our group,7 obtained with independent samples, support the benefits of DBS of the SCG even for patients who have not attained complete remission. However, the risk of clinical aggravation after interruption of stimulation in patients with severe residual symptoms was considered unjustifiable.
Another limitation concerns the assessment measures, because current rating scales, such as the HAMD-17, may not be able to wholly capture the beneficial effects of the intervention. This could have underestimated the effect of electrical stimulation.23 These authors also point out that some behavioural changes induced by DBS, such as increased emotional expression, sociability or the increase of daily activities, may not lead to a clinically important decrease in the rating scale scores. Finally, a 3-month period may be insufficient to fully dissipate carryover effects (i.e., therapeutic benefits could persist in some patients during sham stimulation, and slow gradual improvements could occur after DBS reinitiation). A longer period would have probably heightened differences in favour of the intervention, but it would have been unacceptable for ethical and safety concerns. In light of our results, 3 months seems adequate to reliably test the effects of stimulation of the SGC in patients with TRD.
Conclusion:
Given the trial design, the overall benefits of the present study overcome the potential harmful effects of switching off the electrodes. At this point, the present study constitutes, to our knowledge, the first trial of DBS in patients with TRD using a sham-controlled discontinuation phase. The findings, obtained in a very small sample, highlight the necessity for larger studies, even combining other data sets with similar brain targets. This would help to confirm the results presented herein and to ascertain useful predictors of DBS response in patients with depression.
Acknowledgements
This study was funded by several grants of the Fondo de Investigación Sanitaria (FIS, PI 06/0662, PS 09/00580) from the Instituto de Salud Carlos III, by the Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM) intramural funding (P91G), the ‘VI Plan Nacional I+D+I 2008–2011’, and the ‘Iniciativa Ingenio 2010, programa CONSOLIDER, acción CIBER’. M. Portella is funded by the Ministerio de Ciencia e Innovación of the Spanish Government and by the Instituto de Investigación Carlos III through a “Miguel Servet” research contract (CP10/00393), co-financed by the European Regional Development Fund (ERDF) (2007–2013). J. de Diego-Adeliño is funded by the Instituto de Salud Carlos III through a “Río Hortega” Spanish government research fellowship. The authors thank the Department of Epidemiology of Hospital Sant Pau for comments on an earlier version of this manuscript and the staff of Hospital de la Santa Creu i Sant Pau, who generously provided their time and experience. The authors are extremely grateful to the patients and their families for their invaluable cooperation in this study.
Footnotes
Competing interests: V. Pérez declares educational honoraria from Sanofi-Aventis, Lundbeck, Pfizer, AstraZeneca and Eli Lilly. E. Alvarez has received consulting and educational honoraria from Eli Lilly, Sanofi-Aventis, Lundbeck and Pfizer, and he has participated as main local investigator in clinical trials from Eli Lilly, Bristol-Myers and Sanofi-Aventis and also as national coordinator of clinical trials from Servier and Lundbeck. F. Artigas has received lecture fees from Eli Lilly and Lunbeck A/S on the mechanism of action of antidepressant drugs and consultation fees from Lundbeck A/S. No other competing interests declared.
Contributors: D. Puigdemont, M. Portella, E. Àlvarez and V. Pérez designed the study. R. Pérez-Egea, J. Molet, A. Gironell, and A. Martin acquired the data, which D. Puigdemont, M. Portella, A. Gironell, J. de Diego-Adeliño, A. Martin, R. Rodriguez and F. Artigas analyzed. D. Puigdemont, M. Portella, R. Pérez-Egea and J. Molet wrote the article, which all authors reviewed and approved for publication.
- Received December 24, 2013.
- Revision received March 17, 2014.
- Revision received June 2, 2014.
- Revision received July 28, 2014.
- Revision received October 7, 2014.
- Accepted October 15, 2014.