Abstract
Background: Negative symptoms of schizophrenia can be grouped in 2 dimensions: apathy and diminished expression. Increasing evidence suggests that negative symptoms are associated with altered neural activity of subcortical and cortical regions in the brain reward system. However, the neurobiological basis of the distinct symptom dimensions within negative symptoms is still poorly understood. The primary aim of our study was to examine the neural correlates of the negative symptom dimensions apathy and diminished expression during a reward processing task.
Methods: Patients with schizophrenia and healthy controls underwent event-related fMRI while performing a variant of the Monetary Incentive Delay Task. We assessed negative symptom dimensions using the Brief Negative Symptom Scale.
Results: We included 27 patients and 25 controls in our study. Both groups showed neural activation indicated by blood oxygen–level dependent signal in the ventral striatum during reward anticipation. Ventral striatal activation during reward anticipation showed a strong negative correlation with apathy. Importantly, this effect was not driven by cognitive ability, medication, depressive or positive symptoms. In contrast, no significant correlation with the diminished expression dimension was observed.
Limitations: Although the results remain significant when controlling for chlorpromazine equivalents, we cannot fully exclude potential confounding effects of medication with atypical antipsychotics.
Conclusion: The specific correlation of ventral striatal hypoactivation during reward anticipation with apathy demonstrates a differentiation of apathy and diminished expression on a neurobiological level and provides strong evidence for different pathophysiological mechanisms underlying these 2 negative symptom dimensions. Our findings contribute to a multilevel framework in which apathy and motivational impairment in patients with schizophrenia can be described on psychopathological, behavioural and neural levels.
Introduction
Negative symptoms are core symptoms of schizophrenia and include avolition, anhedonia, asocialty, blunted affect and alogia.1,2 They contribute strongly to poor functional outcome and reduced subjective quality of life.3–5 Despite these consequences, current knowledge about the underlying patho-physiology remains limited, hindering the development of effective treatment strategies.
There is now a consensus that negative symptoms can be grouped into 2 dimensions.2,5,6 First, a motivational dimension, which we refer to as apathy, combines anhedonia, avolition and asociality. Second, a diminished expression dimension includes blunted affect and alogia. Importantly, it has been suggested that different neurobiological mechanisms may underlie these dimensions, which would be highly relevant for identifying specific treatment strategies.1,7,8
Apathy can be defined as reduction of motivation and goal-directed behaviour.9,10 Several behavioural studies have shown that motivational impairments in schizophrenia are associated with dysfunctional processing of reward information in effort-based decision making,11,12 reinforcement learning and during reward anticipation.13,14 On the neural level, the ventral striatum appears to play an important role in coding incentive motivation15,16 or wanting of a reward.17 There is now consistent evidence for striatal dysfunction during reward anticipation in individuals with schizophrenia and their relatives.18–22 Importantly, several research groups have found an association between negative symptom severity and reduced activation in the ventral striatum, although there are some divergent findings.23–30 In addition, an association between negative symptoms and reduced activity in the dorsal striatum during reward processing and cognitive processing tasks have been observed.31,32 Two studies, including our own, suggest that ventral striatal hypoactivation might be more strongly associated with apathy than global negative symptoms.28,29 Data from a positron emission tomography study showing an inverse correlation between dopamine levels within the ventral striatum and apathy support this idea.33 However, in prior studies a clear distinction between neurobiological correlates of apathy and diminished expression was not demonstrated. This might be due to previous research mainly focusing on total negative symptom scores or concentrating on neurobiological correlates of apathy alone. In addition, relatively small samples likely made it difficult to disentangle between apathy and diminished expression.34
The primary aim of the present study was to investigate the specific association between apathy and diminished expression and neural correlates of reward processing in individuals with schizophrenia. We used the new Brief Negative Symptom Scale (BNSS)35 to assess these 2 psychopathological dimensions. To investigate the neural activity during anticipation of reward, we used a variant of the Monetary Incentive Delay Task.15 We hypothesized that apathy but not diminished expression is associated with hypofunction in the ventral striatum during reward anticipation. In addition, since a role for the orbitofrontal cortex (OFC) in the patho-physiology of negative symptoms has been suggested,14 we investigated the association between OFC activation during reward outcome processing and the negative symptom dimensions in an exploratory analysis.
Methods
Participants
Participants with schizophrenia were recruited from outpatient and inpatient units of the Psychiatric Hospital of the University of Zurich or from institutions affiliated with the Psychiatric Hospital, and healthy controls were recruited from the general community. All patients with schizophrenia were clinically stable and received a stable dose of medication (for further details, see Appendix 1, available at jpn.ca).
Diagnosis of schizophrenia was confirmed using a structured Mini-International Neuropsychiatric Interview for DSM-IV.36 We excluded patients with any other DSM-IV Axis I disorder (in particular, current substance use disorder and major depressive disorder), those medicated with lorazepam at a dose higher than 1 mg, those with florid psychotic symptoms (i.e., any positive subscale item score higher than 4 on the Positive and Negative Syndrome Scale [PANSS]37) and those with extrapyramidal side effects (i.e., a total score higher than 2 on the Modified Simpson–Angus Scale [MSAS]38). Healthy controls were screened for any neuropsychiatric disorders using the structured Mini-International Neuropsychiatric Interview36 to ensure that they had no previous or present psychiatric illness. Both patients and healthy controls were required to have a normal physical and neurologic status and no history of major head injury or neurologic disorder.
The project was approved by the local ethics committee of the canton of Zurich. All participants gave written informed consent to participate in the study. The capability to give informed consent of each participant with schizophrenia was evaluated by the treating psychiatrist.
Clinical and neuropsychological assessment
In order to assess negative symptom dimensions, we administered the BNSS35 to participants with schizophrenia. The apathy (motivation and pleasure) dimension score included avolition, asociality and anhedonia, while the diminished expression dimension score included alogia and blunted affect (Appendix 1). Further psychopathological assessment included the Scale for the Assessment of Negative Symptoms (SANS),39 the PANSS, the Calgary Depression Scale for Schizophrenia (CDSS),40 the Global Assessment of Functioning scale (GAF)41 and the Personal and Social Performance Scale (PSP).42 Moreover, both groups performed a neuropsychological test battery assessing different cognitive domains (Appendix 1).
Experimental design and task
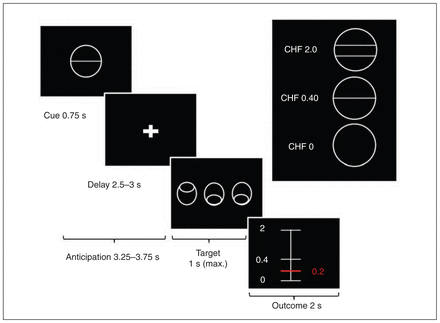
We used a variant of the Monetary Incentive Delay Task (MID)15 with stimuli based on the Cued-Reinforcement Reaction Time Task.43 This variant allowed us to investigate reward anticipation and reward outcome, which depended on the individual task performance (Fig. 1). Before starting the experiment all participants were informed that they would receive the complete amount of money won during the 2 experimental sessions. At the beginning of each trial, 1 of 3 different cues was presented for 0.75 s. The cue indicated the maximum possible amount participants could gain in that trial (i.e., 2 Swiss francs (CHF), 0.40 CHF, or 0 CHF; 1 CHF = $1.08 US). After a delay of 2.5–3 s, the participants had to identify an outlier from 3 presented circles and press a button (either left or right) as fast as possible (varying from 0.32 to 1 s). Immediately, participants were notified of the amount of money they had won (duration of feedback 2 s). Error trials were defined as trials with a wrong response or late response (after 1 s). In all other trials we calculated the actual amount of money to be won for each trial on the basis of the response times of the previous 15 individual trials (Appendix 1, Fig. S1). We used this approach to account for individual differences in response time and thus ensure constant and high rewards in both groups. The maximum amount of money to be won was 50 CHF. Every participant performed 2 training runs, 1 outside and 1 inside the scanner. Excluding the training sessions, the experiment included 2 runs with 36 trials of about 10 s each. The intertrial interval (ITI) was jittered from 1 to 9 s with a mean of 3.5 s to enhance statistical power. In total, 1 run lasted about 6 min. The task was implemented using the MATLAB toolboxes Cogent 2000 and Cogent Graphics.
Variant of the Monetary Incentive Delay Task. First, participants saw 1 of 3 cues indicating the amount of money they could win, if they reacted correctly during the discrimination task. Immediately after target presentation, visual feedback informed the participants about the amount of money they had won during the trial. We used a column ranging from minimal (0 Swiss francs [CHF]) to maximal win amount (CHF 2.0). A red horizontal line indicated the amount of money won during the respective trial.
Functional image acquisition
Imaging data were collected using a Philips Achieva 3.0 T magnetic resonance scanner with a 32-channel SENSE head coil at the MR Zentrum of the Psychiatric Hospital, University of Zurich. Functional MRI scans were acquired in 2 runs with 195 images in each run. We used a gradient-echo T2*-weighted echo-planar image (EPI) sequence with 38 slices acquired in ascending order. Acquired in-plane resolution was 3 × 3 mm2, 3 mm slice thickness and 0.5 mm gap width over a field of view of 240 × 240 mm, repetition time 2000 ms, echo time 25 ms and a flip angle of 82°. The first 5 scans were discarded to eliminate the influence of T1 saturation effects. Slices were aligned with the anterior–posterior commissure. Anatomic data were acquired using an ultrafast gradient echo T1-weighted sequence in 160 sagittal plane slices of 240 × 240 mm resulting in 1 × 1 × 1 mm voxels.
Data analyses
All demographic, clinical, neuropsychological and behavioural data as well as the correlations were analyzed using IBM SPSS Statistics version 22. Normal distribution was tested with the Kolmogorov–Smirnov test. We analyzed fMRI data using SPM8 (Wellcome Department of Cognitive Neurology).
Behavioural data analyses
The main behavioural outcome measure was response time (RT), defined as the time between target presentation and pressing the correct answer button. We performed a 2-way repeated-measures analysis of variance (ANOVA) with group as the between-subjects factor and reward condition (neutral, low, high) as the within-subjects factor. We performed the Mauchly test for the assumption of sphericity; in case of violations we reported Greenhouse–Geyser–corrected degrees of freedom. As post hoc tests for significant main effects, we applied Bonferroni-corrected pairwise comparisons.
Furthermore, we performed correlation analysis between negative symptom factors and reward-related speeding. Reward-related speeding was calculated by subtracting the RT during the neutral condition (CHF 0) from the RT during the high reward condition (CHF 2.0) divided by the mean of these 2 conditions.
Potential group differences in all other behavioural data were investigated using 2-sample t tests. For non-normally distributed data, we applied Mann–Whitney U tests.
Image preprocessing
Functional images were corrected for differences in the time of slice acquisition and motion using the realign and unwarp function of SPM8. A voxel displacement map, calculated from double phase and magnitude field map data, was used to correct for combined static and dynamic distortions. We performed segmentation, bias correction and spatial normalization. Finally, images were smoothed using a 6 mm full-width at half-maximum Gaussian kernel. To assure adequate quality of fMRI data, participants with translational head movement greater than 3 mm or extensive signal dropout in the EPI sequences were excluded. Following previous studies from Van Dijk and colleagues44 and Satterthwaite and colleagues,45 we calculated range of motion as mean relative displacement (MRD) and used it for subsequent analyses.
First- and second-level image analyses
We used a general linear model (GLM) approach to assess our data in an event-related design at the first level. For the 3 different reward anticipation phases, separate regressors were included: anticipation of no reward (CHF 0), anticipation of low reward (CHF 0.40) and anticipation of high reward (CHF 2.0). For the outcome phases we included 1 re-gressor for each condition (3 basic regressors). Additionally, for the low and high reward conditions the 2 outcome regressors were parametrically modulated by the actual outcome amount of each trial. Target presentation (1 regressor) and anticipation, target and outcome phase in error trials (3 regressors) were modelled as regressors of no interest. In total, the first-level model included 12 regressors. The canonical hemodynamic response function was used for convolving all explanatory variables. For reward anticipation we calculated the contrast anticipation of high reward (CHF 2.0) versus anticipation of no reward (CHF 0). For the analysis of the outcome processing phase, we used the parametric modulator for high reward (CHF 2.0). At the second-level analysis, we included the individual contrast images of all participants in a random-effects model. We calculated within-group activation using a 1-sample t test and between-group activation using a 2-sample t test.
Region of interest image analysis
In line with our a priori hypothesis, we defined the ventral striatum as a region of interest (ROI) during anticipation of reward. Coordinates (Montreal Neurological Institute [MNI]) for the ventral striatum were derived from a meta-analysis by Knutson and Greer46 (left: x, y, z = −12, 10, −2; right: x, y, z = 10, 8, 0), who investigated previous fMRI studies using the MID task. This approach was adopted from a recent study by Yip and colleagues,47 who investigated ventral striatal activity in patients with bipolar disorder. In addition to the ventral striatum, we defined the medial orbitofrontal cortex (mOFC) as an ROI for analysis of activation during the outcome phase. This ROI was constructed as an anatomic voxel mask with the Individual Brain Atlases tool in SPM (IBASPM 71)48 implemented in the Wake Forest University Toolbox.49 The statistical thresholds were set at p = 0.05 at the voxel level, with a family-wise error (FWE) rate correction of p = 0.05 for multiple comparison in each ROI. Mean percent signal changes were extracted for all voxels in the ventral striatum and mOFC using the REX toolbox (http://web.mit.edu/swg/software.htm).
Correlation analysis
We tested our main hypothesis by calculating bivariate Pearson correlations (r) between negative symptoms (apathy and diminished expression) and percent signal change in the ventral striatum and mOFC. Partial correlations were calculated to control for potential confounding variables. Finally, we performed the Steiger test for dependent correlation coefficients to test for potential differences between these correlations.50
Results
Sample characteristics
Initially, 30 patients with schizophrenia and 28 healthy control participants were included in our study; however, 5 participants (2 schizophrenia, 3 control) were excluded because of head movement, and 1 participant with schizophrenia was excluded owing to signal dropout in functional images, resulting in a final sample of 27 patients (11 outpatients and 16 inpatients) and 25 healthy controls. Participant characteristics, clinical data and group comparisons are summarized in Table 1. In line with previous research, the 2 negative symptom dimensions apathy and diminished expression correlated significantly (r = 0.45, p < 0.02).12 Mean MRD did not differ significantly between healthy controls and patients with schizophrenia (0.11 ± 0.03 v. 0.11 ± 0.04, p = 0.43).
Demographic, psychopathological and clinical characteristics of study participants
Behavioural data
Regarding RT, the repeated-measures ANOVA revealed no significant main effect of group (F1,50 = 2.6, p = 0.12) but a significant main effect of reward (F1.4, 69 = 34.0, p < 0.001). Post hoc pairwise comparison of the RT showed significant differences among all 3 conditions (all p < 0.001). Participants were significantly faster in the low versus no reward, high versus low reward and high versus no reward conditions (Appendix 1, Fig. S2). These results indicate intact reward-related speeding in both groups. There was no significant group × reward interaction effect (F1.4, 69 = 0.65, p = 0.47). Furthermore, reward-related speeding did not correlate significantly with apathy (r = −0.22, p = 0.26) or diminished expression (r = 0.02, p = 0.90). Additionally, to control for differences in cognition, we performed an analysis of covariance with the composite cognitive ability score as a covariate, which did not change the significance levels in the repeated-measures ANOVA.
Because of low error rates in all 3 conditions we used total error rates for group comparison. We did not find any differences between healthy controls and patients with schizophrenia (U = 313.5, p = 0.66; Table 2). Finally, differences in total gain were significant, but small (t = 2.1, p = 0.040; Table 2).
Behavioural results of the variant of the Monetary Incentive Delay task
Functional imaging data
Anticipation of reward: ventral striatum
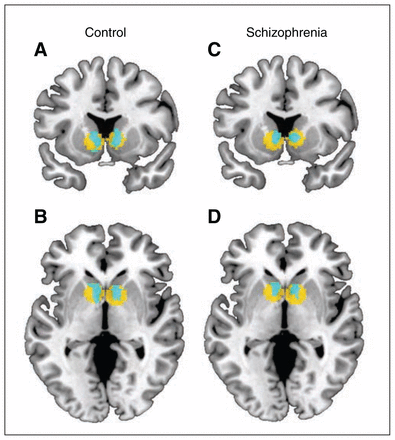
We focused on brain activation during anticipation of high reward versus no reward. Our a priori defined ROI was the ventral striatum. Healthy controls (left: cluster size = 428, t = 5.72; right: cluster size = 398, t = 5.60, both p < 0.05, FWE-corrected) and patients with schizophrenia (left: cluster size = 259, t = 5.44; right: cluster size = 361, t = 5.42, both p < 0.05, FWE-corrected) showed significantly stronger activation in the ventral striatum during anticipation of high reward than anticipation of no reward (Fig. 2). However, in the group comparison we did not find any significant differences during anticipation of high reward versus no reward either in the left or in the right ventral striatum. Importantly, all significance levels remained unchanged when including the composite cognitive ability score as a covariate.
Within-group activation maps of the contrast anticipation of high reward versus no reward in the ventral striatum (p < 0.05, family-wise error–corrected). The within group t maps (blue) were overlaid on the region of interest (yellow). (A) Coronal and (B) axial contrast images of healthy controls. (C) Coronal and (D) axial contrast images of participants with schizophrenia.
Correlation between anticipation of reward and negative symptoms
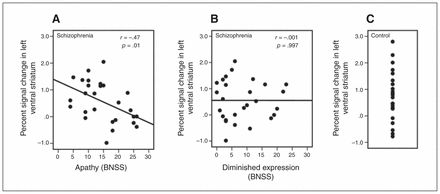
Regarding our primary hypothesis we found a highly significant negative correlation between percent signal change in the ventral striatum during reward anticipation and apathy in the left ventral striatum (r = −0.47, p = 0.014; Fig. 3) and in the right ventral striatum (r = −0.38, p = 0.05). Thus, a higher apathy score was associated with less ventral striatal activation during anticipation of high reward in relation to anticipation of no reward. In contrast, no significant correlation between diminished expression and percent signal change in the ventral striatum during reward anticipation was observed in the left ventral striatum (r = −0.001, p > 0.99; Fig. 3) and right ventral striatum (r = 0.09, p = 0.65). These correlation coefficients were significantly different from each other (left ventral striatum: tDiff = 2.40, p = 0.017; right ventral striatum: tDiff = 2.32, p = 0.020); apathy was more strongly associated with reduced ventral striatal activation than diminished expression. Additionally, we performed whole brain voxelwise exploratory correlation analyses, revealing no additional cluster showing significant correlation of apathy or diminished expression with activation during reward anticipation.
Bivariate Pearson correlation (including significance test) of (A) apathy and (B) diminished expression with percent signal change in the left ventral striatum in participants with schizophrenia. (C) Percent signal change in the left ventral striatum in healthy controls. BNSS = Brief Negative Symptom Scale.
None of the potential confounding variables, such as chlorpromazine equivalents (left ventral striatum: r = −0.05, p = 0.82; right ventral striatum: r = 0.01 p = 0.95), depressive symptoms as per the CDSS (left ventral striatum: r = 0.45, p = 0.02; right ventral striatum: r = 0.34, p = 0.08), positive symptoms as per the PANSS positive factor (left ventral striatum: r = −0.23, p = 0.26; right ventral striatum: r = −0.17, p = 0.40), cognition as per the composite cognitive ability score (left ventral striatum: r = 0.05, p = 0.80; right ventral striatum: r = 0.12, p = 0.50) and total amount of gain (left ventral striatum: r = 0.22, p = 0.27; right ventral striatum: r = 0.28, p = 0.16), showed a significant negative correlation with ventral striatal activation. Furthermore, we computed partial correlations to include these potential confounding variables in our main analyses (i.e., the correlations between ventral striatal activation and apathy/diminished expression). The association between apathy and percent signal change remained significant in the left ventral striatum (r = −0.52, p = 0.014), but not in the right ventral striatum (r = −0.38, p = 0.08), when including all covariates stated above. In contrast, the correlation between diminished expression and percent signal change in the ventral striatum remained nonsignificant (left ventral striatum: r = −0.16, p = 0.46; right ventral striatum: r = 0.15, p = 0.50). Importantly, neither ventral striatum activation nor apathy scores were related to the range of motion during data acquisition (MRD; Appendix 1, Table S1). Additionally, an exploratory analysis of correlations between ventral striatum activation and all BNSS items and additional psychopathological assessment measures were performed (Appendix 1, Table S2 and S3). Interestingly, these data revealed that the association of apathy with reduced ventral striatum activation was strongest for the avolition subscale (left ventral striatum: r = −0.53, p = 0.005; right ventral striatum r = −0.44, p = 0.021).
Reward outcome processing: ventral striatum, mOFC
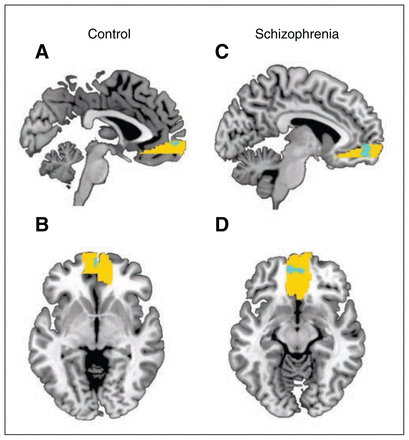
Significant ventral striatal activation related to reward magnitude were observed in patients with schizophrenia (left ventral striatum: cluster size = 40, t = 4.4; right ventral striatum: cluster size = 31 t = 4.2, both p < 0.05, FWE-corrected) but not in healthy controls. Group comparison showed a significant difference in ventral striatal activation in the right ventral striatum (cluster size = 38, t = 4.4, p = 0.011, FWE-corrected). In contrast, healthy controls and patients exhibited significant activation in the mOFC associated with reward magnitude (controls: cluster size = 39, t = 4.5; patients: cluster size = 400, t = 4.9, both p < 0.05, FWE corrected; Fig. 4). The group comparison did not reveal any significant differences in the reward responses in the mOFC. Importantly, all significance levels remained unchanged when including the composite cognitive ability score as a covariate.
Within-group activation maps of the parametric modulator of high reward outcome processing in the medial orbitofrontal cortex (i.e., activation associated with the amount of reward actually received, p < 0.05, family-wise error–corrected). The within group t maps (blue) were overlaid on the region of interest (yellow). (A) Sagittal and (B) axial contrast images of healthy controls. (C) Sagittal and (D) axial contrast images of participants with schizophrenia.
Correlation between outcome processing and negative symptoms
There were no significant correlations between the 2 negative symptom dimensions and the ventral striatum or mOFC responses to reward magnitude. Furthermore, we observed no significant association between reduced activations of these regions and positive or depressive symptoms.
Discussion
In line with our a priori hypothesis, we observed a strong association of reduced striatal activation during reward anticipation with apathy but not with diminished expression. To our knowledge, this is the first study showing a differentiation of apathy and diminished expression on a neurobiological level. At a behavioural level, previous work by our group and others using effort-based decision making has already provided evidence for different pathophysiological mechanisms underlying the 2 main negative symptom dimensions.11,12,51 In a recent study, motivational deficits in schizophrenia, as measured by a progressive ratio task, were associated with clinical amotivation and neural hypoactivation of the ventral striatum.30 The present study extends this work by providing a specific link between the clinical expression of apathy and dysfunctional striatal reward anticipation, which strongly supports the hypothesis of different neural bases for apathy and diminished expression.34 In this context, our present findings highlight the importance of assessing both negative symptom domains separately when investigating the neural basis of negative symptoms.1,7,8,34
For the study of apathy it is crucial to account for secondary negative symptoms, which are related to medication side effects, as well as positive psychotic and depressive symptoms.52,53 We aimed to reduce the potential influence of secondary negative symptoms on our main findings using the following approach. First, we excluded patients at high risk of displaying secondary negative symptoms (i.e., all patients were clinically stable and showed no signs of major depression, no more than moderate positive symptoms and no signs of any extrapyramidal side effects). Second, none of the measures for potential causes of secondary negative symptoms was associated with reduced ventral striatal activation. Third, the negative correlation between apathy and ventral striatal activation remained highly significant after controlling for all covariates. In addition, the association of apathy and reduced ventral striatal activation was not influenced by cognitive ability.
Importantly, the negative association with apathy was strongly related to the ventral striatum during reward anticipation. A correlation of apathy with activation related to the magnitude of received reward was observed neither in the ventral striatum nor in the mOFC. Preclinical studies have consistently emphasized that neurotransmission in the midbrain dopamine system, particularly the projections to the dorsal and ventral striatum, mediates incentive motivation or wanting of reward.17,34,54 Furthermore, dopamine depletion or antagonism in the ventral striatum leads animals to choose a high-effort option for larger rewards less often even though preferences for larger rewards remain intact in the absence of effort requirements.34,55 Conversely, administration of amphetamine in the ventral striatum or genetic mutations amplifying the extracellular dopamine levels contribute to an increase in wanting and motivated behaviour.56,57 Human fMRI studies have shown a critical role of ventral striatal activation during reward anticipation and its association with negative symptoms in patients with schizophrenia.23,25,27–29 Now, our findings directly demonstrate for the first time that the association of ventral striatum hypofunction with apathy is significantly stronger than the association with diminished expression. Hence, we argue that hypoactivation in the ventral striatum reflects a disrupted wanting of reward, which contributes to a reduction of motivated behaviour and is eventually expressed as apathy on the clinical and psychopathological level.
At the group level, there were no significant differences between patients with schizophrenia and healthy controls in neural activation in the ventral striatum during reward anticipation, which is in line with the results of most but not all previous studies.18,24,25,28,29 One potential explanation of this observation is the fact that all patients in our study were treated with atypical antipsychotics. Thus, our findings are in line with those of Schlagenhauf and colleagues,24 emphasizing an improvement of reduced ventral striatal activity in patients on atypical but not on typical antipsychotics and of Juckel and colleagues,18 showing reduced ventral striatum activation in patients treated with typical but not atypical antipsychotics. Furthermore, our results suggest an alternative explanation. We observed that neural activity in the ventral striatum during reward anticipation is negatively correlated with the severity of apathy in patients with schizophrenia. Thus, patients with low expression of apathy may have intact neural activity in the ventral striatum during reward anticipation, which in turn limits the observed difference in group comparisons.
Our finding of an intact mOFC response during receipt of reward is in line with those of previous imaging studies.19,27,28 Furthermore, we did not find any association between mOFC activation and the apathy dimension. This could be interpreted as an intact in-the-moment experience or as a hedonic response in apathy, as recently proposed by various authors.14,58 However, it has to be acknowledged that our task was not designed to assess whether a prefrontal hypofunction contributes to apathy in addition to ventral striatal dysfunction.
Limitations
Although the present results provide strong evidence of the association of ventral striatal dysfunction during reward anticipation and apathy in patients with schizophrenia, it is important to note some limitations. First, to fully support the hypothesis of different neural bases of apathy and diminished expression, a double dissociation would have to include neural correlates of diminished expression in addition to those for apathy presented here. Second, although in our analyses antipsychotic dose did not have any statistical effect, it would be important to investigate whether our findings can be generalized to unmedicated patients and patients treated with first-generation antipsychotics. Third, screening for potential causes of secondary negative symptoms led to low variance in positive and depressive symptoms, which might be responsible for the lack of an association of these symptoms with reduced ventral striatal activation during reward anticipation. Thus, specificity with respect to these symptom dimensions cannot be inferred from the present study. Likewise, the positive correlation of depressive symptoms with ventral striatal activity during reward anticipation has to be considered in the light of this limitation and should be interpreted with caution. Concerning the analyses of the reward outcome, we have to acknowledge that the MID task often does not produce robust neural signal of the ventral striatum during the outcome phase, as observed in healthy controls in the present study. Therefore, our finding of a lack of correlation between ventral striatal activation related to reward outcomes and apathy should be interpreted with caution.
Conclusion
The specific correlation of ventral striatal hypoactivation and apathy provides strong evidence for different underlying pathophysiological mechanisms of the 2 negative symptom domains. Our findings contribute to a multilevel framework in which apathy and motivational impairment can be described on psychopathological, behavioural and neural levels, as proposed in the Research Domain Criteria approach.59 Ventral striatal hypoactivation could be a potential neuroimaging marker for pharmacological treatment trials aiming at negative symptoms, which could help to further elucidate the mechanisms underlying treatment effects.
Acknowledgments
This study was supported by the Swiss National Science Foundation (Grant No. 105314_140351 to S. Kaiser). P.N. Tobler was supported by the Swiss National Science Foundation (PP00P1_128574, PP00P1_150739, and CRSII3_141965). The authors thank Dr. Philipp Staempfli and Dr. Benedikt Habermeyer for their excellent support, and all patients and healthy volunteers for their participation.
Footnotes
Competing interests: E. Seifritz has received grant support from Lundbeck and has served as a consultant and/or speaker for Astra-Zeneca, Otsuka, Eli Lilly, Janssen, Lundbeck, Novartis, Pfizer, Roche, and Servier. S. Kaiser has received speaker honoraria from Roche, Lundbeck, Janssen and Takeda. He receives royalties from Schuhfried for cognitive test and training software. None of these activities is related to the present study. None declared by any other authors.
Contributors: M. Kirschner, O.M. Hager, E. Seifritz, P.N. Tobler and S. Kaiser designed the study. M. Kirschner, O.M. Hager, M. Bischof, M.N. Hartmann and A. Kluge acquired the data, which M. Kirschner, O.M. Hager, M.N. Hartmann, P.N. Tobler and S. Kaiser analyzed. M. Kirschner and S. Kaiser wrote the article, which all authors reviewed and approved for publication.
- Received December 26, 2014.
- Revision received April 27, 2015.
- Accepted May 6, 2015.