Abstract
Background: Bulimia nervosa is a severe psychiatric syndrome with uncertain pathogenesis. Neural systems involved in sensorimotor and visual processing, reward and impulsive control may contribute to the binge eating and purging behaviours characterizing bulimia nervosa. However, little is known about the alterations of functional organization of whole brain networks in individuals with this disorder.
Methods: We used resting-state functional MRI and graph theory to characterize functional brain networks of unmedicated women with bulimia nervosa and healthy women.
Results: We included 44 unmedicated women with bulimia nervosa and 44 healthy women in our analyses. Women with bulimia nervosa showed increased clustering coefficient and path length compared with control women. The nodal strength in patients with the disorder was higher in the sensorimotor and visual regions as well as the precuneus, but lower in several subcortical regions, such as the hippocampus, parahippocampal gyrus and orbitofrontal cortex. Patients also showed hyperconnectivity primarily involving sensorimotor and unimodal visual association regions, but hypoconnectivity involving subcortical (striatum, thalamus), limbic (amygdala, hippocampus) and paralimbic (orbitofrontal cortex, parahippocampal gyrus) regions. The topological aberrations correlated significantly with scores of bulimia and drive for thinness and with body mass index.
Limitations: We reruited patients with only acute bulimia nervosa, so it is unclear whether the topological abnormalities comprise vulnerability markers for the disorder developing or the changes associated with illness state.
Conclusion: Our findings show altered intrinsic functional brain architecture, specifically abnormal global and local efficiency, as well as nodal- and network-level connectivity across sensorimotor, visual, subcortical and limbic systems in women with bulimia nervosa, suggesting that it is a disorder of dysfunctional integration among large-scale distributed brain regions. These abnormalities contribute to more comprehensive understanding of the neural mechanism underlying pathological eating and body perception in women with bulimia nervosa.
Introduction
Bulimia nervosa is a severe psychiatric syndrome that usually begins during adolescence in girls. It is characterized by episodic binge eating followed by purging behaviours to avoid weight gain. The disorder was thought to be the product of a variety of sociocultural, psychological and biological factors;1 however, its neurobiological underpinnings remain largely unknown, hindering the development of new evidence-based treatments.
Neuroimaging techniques, especially functional MRI (fMRI), provide powerful tools to explore the pathophysiology of bulimia nervosa. Researchers have mainly used task-based paradigms, such as stimuli related to food/state, body and impulsive control, to elicit the neural processes associated with bulimia nervosa. The neural profile observed in patients with bulimia nervosa appears to be subserved by dysfunctions of a variety of cortical and subcortical regions.
Specifically, reported abnormalities primarily pointed to the ventral limbic and paralimbic systems, particularly for the ventral striatum, amygdala, insula, anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC), which are important for reward and emotional processing and interoception;2–8 the occipital cortex, which mediates visual perception and processing;9–11 the dorsal and medial prefrontal cortex (PFC), which subserve impulsive inhibition function;12,13 and the somatosensory regions.8 Dysfunction in these regions has been proposed to comprise key neural mechanisms underlying altered reward and emotional sensitivity,2,4–7 interoceptive deficits3 and self-regulatory difficulties,12,13 especially in response to eating- and body-related stimuli. These multidimensional pathological features collectively induce the body image distortion and binge eating and purging behaviours characteristic of bulimia nervosa. A few studies have highlighted an involvement of abnormal intrinsic functional connectivity (iFC) of the somatosensory network,14 ACC and precuneus15 in the disturbances of body image processing of bulimia nervosa.
Despite increased knowledge of neuroimaging patterns, little is known regarding possible disruptions of the topological organization of whole-brain functional networks in bulimia nervosa. The brain is composed of complex biological systems that integrate signals from multiple regions to facilitate effective information processing.16,17 Graph theory provides a powerful means of quantitatively describing the topological organization of brain connectivity. A number of topological properties, including small-worldness, network hubs and modularity, have been characterized in the human brain.17 These network properties are disrupted under pathological conditions, such as schizophrenia,18 depression19 and anorexia nervosa.20 Although a few studies14,15 have used resting-state fMRI to map the brain connectivity in patients with bulimia nervosa, they all adopted a seed-based analysis strategy examining connectivity between predefined regions — a potentially biased approach lacking a global brain perspective. Because bulimia nervosa involves disturbances of a broad range of neural processes from basic sensory and visual processing to higher-level cognition functions,21 it is likely that this disorder affects information exchange across large-scale brain networks. A network-based statistics (NBS) analysis22 that incorporates multiple brain networks could facilitate the examination of neurobiological alteration in a broader context, which may contribute to a better understanding of the behavioural complexity of bulimia nervosa.
With these knowledge gaps in mind, the present study was undertaken to determine if patients with bulimia nervosa would show disrupted topological organization of functional brain networks. Specifically, we collected resting-state fMRI data from patients with bulimia nervosa and healthy controls and systematically analyzed their topological properties and network interactions using graph theory and NBS. We hypothesized that patients with bulimia nervosa would show abnormal topological properties in functional brain networks and that the functional disturbances would be apparent in the cortical and subcortical regions involved in reward and emotional processing, sensorimotor and visual perception, and interoception, based on potential relevance of these neural functions to bulimia nervosa.2–15 We also examined the behavioural/symptom correlates of these network properties.
Methods
Participants
We recruited women with bulimia nervosa and healthy control women. Those with bulimia nervosa were recruited from the outpatient and inpatient services of Peking University Sixth Hospital. Controls were recruited via local advertisements. The diagnosis of bulimia nervosa was made according to DSM-IV criteria. Patients were required to be 16–30 years old, to currently have both binge eating and compensatory behaviours and to have been free of any psychotropic medications for at least 1 month before the study. We excluded those with current comorbid psychiatric disorders according to DSM-IV criteria, including depressive disorder and anxiety, in order to avoid potential confounding effects on the results. Healthy controls were required to have a body mass index (BMI) of 17.5–25 kg/m2, normal menstrual cycles and no lifetime history of any psychiatric disorder. We conducted a face-to-face interview for each healthy control participant using the Mini-International Neuropsychiatric Interview (MINI) to exclude those with any psychiatric disorder.23
All participants completed the Eating Disorder Inventory-1 (EDI-1) questionnaire, the 17-item Hamilton Rating Scale for Depression (HAMD-17) and the Hamilton Anxiety Rating Scale (HAM-A) on the day of scanning. The EDI-1 is a 64-item self-report questionnaire of psychological traits that are clinically relevant in individuals with eating disorders.24 Participants respond on a 6-point Likert scale (with responses ranging from “always” to “never”). This study reports the scores of EDI-1 subscales of drive for thinness, bulimia, body dissatisfaction and interoceptive deficits. The Institutional Review Board of Peking University Sixth Hospital approved this study, and all participants provided written informed consent.
MRI acquisition
Images were acquired using a Siemens 3 T scanner. The resting-state functional images were obtained using an echo- planar imaging sequence with the following parameters: repetition time (TR) 2000 ms, echo time (TE) 30 ms, flip angle 90°, 64 × 64 matrix, slice thickness 4.0 mm, gap 0.8 mm, 30 slices, duration 7 minutes. In addition, we obtained T1-weighted images using a magnetization-prepared rapidly acquired gradient-echo (MPRAGE) sequence under the following parameters: TR 2300 ms, TE 3.01 ms, 256 × 256 matrix, flip angle 9°. We asked the participants to keep their eyes closed without falling asleep and to move as little as possible. Through confirmation after scanning, no participants reported discomfort or falling asleep during scanning, and no participants showed obvious structural damage based on conventional MRI scans.
Data preprocessing
The resting state fMRI images were preprocessed using Data Processing Assistant for Resting-State fMRI (DPARSF; http://rfmri.org/DPARSF), which is based on Statistical Parametric Mapping version 12 (SPM12; www.fil.ion.ucl.ac.uk/spm). After removing the first 10 volumes, we corrected the remaining 200 volumes for different signal acquisition times. The functional volumes were motion-corrected using a 6-parameter rigid-body transformation. Then, the nuisance signals (including Friston 24-parameter model25,26 of head-motion parameters, cerebrospinal fluid signal, white matter signal and linear trend) were regressed out. Derived images were normalized to Montreal Neurological Institute (MNI) space (3 mm3 isotropic) using the Diffeomorphic Anatomic Registration with the Exponentiated Lie (DARTEL) algebra tool. Then, the transformed images were band-pass filtered (0.01–0.1 Hz).
Given a possible confounding effect of micromovements on iFC,27 we calculated the framewise displacement (FD) values for each participant using the Jenkinson fomula,28 which could reflect the temporal derivative of the movement parameters.26
Network construction
The connectome graph is composed of distinct brain regions (nodes) and their functional interactions (edges). The whole brain was first parcellated into 90 cortical and subcortical regions of interest (45 for each hemisphere; Appendix 1, Table S1, available at jpn.ca/160183-a1), as defined by the automated anatomic labelling (AAL) atlas.29 The mean time series of each region was extracted by averaging the time series of all voxels within that region. We estimated Pearson correlation coefficients for each pair of regions, and they were transformed to Fisher z-scores to create the connectivity matrix for each participant. The correlation matrices were further thresholded into binary networks by applying a density threshold (the number of edges divided by the maximum possible number of edges; also called “sparsity threshold” in some studies) to normalize the number of edges among the graphs. Applying a 10% threshold to a correlation matrix means assigning the top 400 edges (10% of the maximum 4005 edges) to 1 and assigning the remaining edges to 0.
Network analysis
Based on the constructed brain networks, we examined both the global and regional topological properties of brain graphs. At the global level, we examined the small-world parameters involving the clustering coefficient (Cp), characteristic path length (Lp), and their normalized versions using a random network (γ, λ). At the regional level, we examined the degree centrality and efficiency for each node. A wide range of density thresholds (10% ≤ density ≤ 34%, step of 1%) was chosen to allow prominent small-world properties in brain networks to be observed, similar to a previous study.19 For each metric, the area under the curve (AUC) for this density range was calculated as representative to avoid the results being dependent on a few densities. See Appendix 1, Table S2, and the study by Rubinov and Sporns30 for the definitions and interpretations of these network measures.
Statistical analysis
Between-group differences
To determine whether there are significant group differences in the network properties, we performed 2-sample t tests on AUC values of each network metric (small-world parameters and regional centrality measures), with age, education and mean values of FD as covariates. We used MATLAB’s Lilliefors test (i.e., lillietest) to determine if the measures were normally distributed and found none of them rejected the null hypothesis (normally distributed) at the 5% significance level. Thus, using a t test is appropriate in such a condition. We identified the brain regions showing significant between-group differences in at least 1 nodal metric (node degree or efficiency; p < 0.05, uncorrected, as an exploratory analysis). Further, we used NBS to localize specific pairs of brain regions in which iFC was altered in patients with bulimia nervosa. To facilitate data interpretation, we sorted connections based on functional hierarchy31 (i.e., primary sensorimotor, unimodal, heteromodal, paralimbic, limbic and subcortical).
Associations between network measures and clinical variables
The partial correlation analysis was performed between the network measures showing between-group differences and clinical variables, including current BMI, illness duration, HAMD and HAM-A scores, and EDI-1 scores for body dissatisfaction, bulimia, drive for thinness and interoceptive awareness, in patients with bulimia nervosa, using age, education and mean FD as covariates.
Validation: reproducibility
We addressed possible concerns regarding the generalizability of our findings to other studies by varying several factors:1 network types, head-motion scrubbing and brain parcellation approach. First, we implemented weighted-network analyses to assess the stability of our findings across different network types.2 In regards to head-motion scrubbing, in the main analysis, we added motion parameters from the Friston 24-parameter model into regression at the individual level and mean FD as a covariate in group analysis to control for head motion. To assess whether residual motion biased group differences, we reprocessed data with individual-level head motion scrubbing instead of group-level motion covariation. “Bad” time points were identified using a threshold of FD > 0.2 mm as well as 1 back and 2 forward neighbours, as performed previously.32 Then each “bad” time point was modelled as a separate regressor in the nuisance covariate regression models. Topological properties and network connectivity were compared based on the scrubbed data; we excluded 6 patients and 9 controls from this validation analysis because more than 50% of the time points were scrubbed.3 For the brain parcellation approach, we redefined nodes using a high-resolution atlas33 with 1024 nodes to examine the reproducibility of our results across different parcellation strategies. We mainly examined the reproducibility of the results of small-world parameters and nodal strength measures.4 We also tested our results using nonparametric permutation tests. Briefly, we calculated the between-group difference in the mean value of each network metric. For each network metric, we then randomly reallocated all the values into 2 groups and recomputed the mean differences between the 2 randomized groups. This randomization procedure was repeated 10 000 times, and the 95th percentile points of each distribution were used as the critical values for a 1-tailed test of the null hypothesis with a probability of type I error of 0.05. Multiple regression analyses were applied to remove the confounding effects of age, education and mean values of FD for each network metric.
Results
Participant characteristics
We recruited 48 women with bulimia nervosa (mean age 22.0 ± 3.4 yr) and 45 healthy control women (mean age 23.1 ± 3.4 yr). Of those recruited, 4 patients and 1 control participant had a mean FD > 0.2 mm, translation > 2 mm, or rotation > 2° and were excluded, leaving data from 44 patients and 44 controls for further analysis. There were no significant differences in age, years of education, or current BMI between patients with bulimia nervosa and healthy controls; however, those with bulimia nervosa showed significantly higher scores than controls on the EDI-1, HAMD-17 and HAM-A (Table 1). Among the 44 women with bulimia nervosa, 17 had a history of anorexia nervosa.
Demographic data in patients with bulimia nervosa and healthy controls
Small-world properties
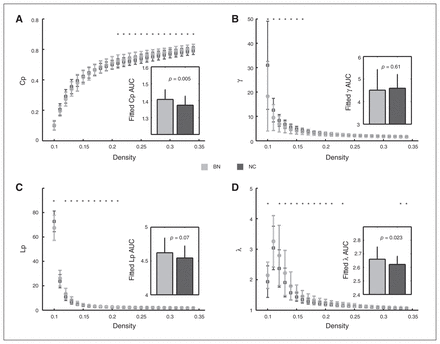
For the AUC across density range of 10% to about 34%, we found significantly increased local clustering (indexed by Cp) in patients with bulimia nervosa compared with healthy controls, which was contributed mostly by higher densities (20% to about 34%; Fig. 1A), whereas the difference in local clustering was not significant after the Cp was normalized by matched random networks (indexed by γ; Fig. 1B). For shortest path length, patients with bulimia nervosa showed a trend of increased Lp, mostly contributed by lower densities (12% to about 24%; Fig. 1C). Interestingly, after being normalized by matched random networks, the increase in path length (indexed by λ) became significant (Fig. 1D).
Between-group differences in small-world properties of functional brain networks across a wide range of density thresholds. The inserts represent the differences of the area under curve (AUC) for each topological parameter across the density range of 10% to about 34%. Error bars denote standard deviations. Black asterisks indicate where the differences between groups are significant at a given density threshold (t test, p < 0.05). A) Clustering coefficient (Cp); B) normalized clustering coefficient, γ; C) shortest path length (Lp); D) normalized shortest path length, λ.
Nodal characteristics
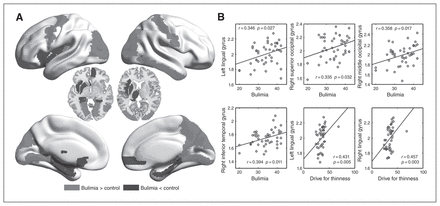
Compared with the healthy controls, patients with bulimia nervosa showed increased nodal strength in the primary sensorimotor (left precentral gyrus, right postcentral gyrus) and unimodal visual association (bilateral superior occipital cortex, bilateral middle occipital cortex, left cuneus, bilateral lingual gyrus and right inferior temporal gyrus) regions, as well as the right precuneus, but decreased nodal strength in the medial orbital frontal gyrus (right middle OFC, left olfactory cortex), medial temporal lobe (left hippocampus, bilateral parahippocampal gyrus [PHG]) and several subcortical regions (left insula, left amygdala, left putamen and left thalamus; Fig. 2 and Appendix 1, Table S3).
Between-group differences in nodal strength (nodal degree or efficiency) and their associations with clinical variables in patients with bulimia nervosa. A) The medium grey areas indicate increased nodal strength in patients with bulimia nervosa compared with healthy controls, and the darker grey areas represent the opposite. B) Scatter plots of nodal metrics against clinical variables.
Network connectivity
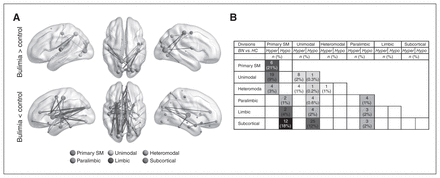
The NBS analysis identified a significantly altered network comprising seemingly disparate themes of both hyper- and hypoconnectivity in women with bulimia nervosa. Analyses sorting abnormal iFC based on functional hierarchy31 showed that the hyperconnectivity extended across the functional divisions of the primary sensorimotor, unimodal association and heteromodal association systems, with primary sensorimotor and unimodal visual association regions having the highest proportions of affected connections (Fig. 3 and Appendix 1, Table S4), whereas the hypoconnectivity was associated with subcortical (caudate, putamen, thalamus), limbic (amygdala, hippocampus) and paralimbic (OFC, ACC, PHG) system regions, particularly for connectivity between subcortical regions and sensorimotor and unimodal visual association regions (Fig. 3 and Appendix 1, Table S5).
Between-group differences in whole-brain functional connectivity. A) Both hyperconnectivity and hypoconnectivity in patients with bulimia nervosa (BN) compared with healthy controls (HC). The size of the spheres indicates the number of increased or decreased connectivities with a node. B) The table summarizes the absolute number and percentage of intrinsic functional connectivity within and between functional divisions showing significant differences between patients and controls. White cells represent the absence of significant intrinsic functional connectivity. Hyper cells represent hyperconnectivity (bulimia nervosa > healthy control), whereas Hypo cells represent bulimia nervosa–related hypoconnectivity (bulimia nervosa < healthy control). Shading decreases proportionally from the highest percentage (21%) to the lowest (0%). SM = sensorimotor.
Clinical associations
There were no significant correlations between small-world measures and clinical variables. For the nodes showing altered degree or efficiency, we found significant positive correlations between nodal strength of several occipital and temporal lobe regions and bulimia (Fig. 2B). The significant positive correlation was also detected between nodal strength of the bilateral lingual gyrus and drive for thinness, but we can see from Figure 2 that the correlations might be driven by an outlier, which may indicate that the inference of clinical significance of this finding in the following discussion should be taken cautiously. The iFC in a number of the connectivity pairs between the subcortical (thalamus, putamen, caudate) and paralimbic (ACC) regions and sensorimotor and unimodal visual regions showed significant negative correlations with bulimia and drive for thinness, whereas several connectivity pairs between the thalamus and unimodal visual regions were significantly positively correlated with BMI (Table 2).
Significant correlations observed between the intrinsic functional connectivity aberrations and clinical variables in women with bulimia nervosa
Reproducibility of the findings
For the measures of small-world properties, the results of increased clustering and path length could also be observed after scrubbing and accounting for the effects of parcellation strategies. Specifically, patients with bulimia nervosa showed significant increases in the AUC of both Cp and λ with head-motion scrubbing as well as significant increases in the AUC of λ and an increased trend in Cp with the 1024-nodes atlas (Appendix 1, Fig. S1). Notably, we observed an increased trend in λ, but no significant group differences in clustering measures during the analysis with weighted networks (Appendix 1, Fig. S1). However, we thought that the results of binary networks in the main analysis may be more reliable, given previous findings showing a trend of greater reliability of the binary node definition of a network compared with its weighted node definition.34,35 The results of group differences of the small-world properties could also be repeated after nonparametric permutation tests (Cp: p = 0.005; γ: p = 0.61; Lp: p = 0.07; γ: p = 0.023).
For the measures of nodal degree or efficiency, most of the results were preserved after scrubbing and accounting for the effects of parcellation strategies and network types (Appendix 1, Fig. S2A–C). Of note, we found nodal abnormalities in broader regions with the 1024-nodes atlas (Appendix 1, Fig. S2C). A possible reason may be that the topological organizations are largely dependent on spatial parcellation strategies; analysis with high-resolution templates could provide more detailed information about the network changes.36 The results of group differences of nodal degree or efficiency after nonparametric permutation tests were very similar to those obtained with 2-sample t tests (Appendix 1, Table S4).
Discussion
The present study examined the topological organization of whole brain networks in women with bulimia nervosa. Basically consistent with the previous hypothesis, we found altered topological properties and networks in women with the disorder, mainly including increased clustering and path length; higher nodal strength in sensorimotor and visual regions and the precuneus, but lower nodal strength in several subcortical regions (insula, amygdala, putamen, thalamus), hippocampus, PHG and OFC; and both hyper- and hypoconnectivity, with the hyperconnectivity primarily involving sensorimotor and unimodal visual association regions and the hypoconnectivity being associated with subcortical, limbic and paralimbic system regions, particularly for connectivity between subcortical regions and sensorimotor and unimodal visual regions.
The human brain is a complex network with some fundamental organizational principles;17 among them, the small-worldness is a very important attribute.17 According to 2 basic properties, the clustering coefficient and path length, a network can be classified into regular, random and small-world networks.17 Regular networks are characterized by high clustering and long path length, indicating good local but poor global efficiency,37 whereas random networks have low clustering and short path length, indicating the opposite situation.37 A small-world network possesses a combination of high clustering and short path length. This economical yet efficient communication architecture is crucial for normal brain functions,38 such as cognitive abilities.38 We found increased clustering and path length in women with bulimia nervosa, suggesting increased local but decreased global efficiency and a shift toward regular network, which has been characterized in several other psychiatric diseases.39,40 Although the exact mechanism remains unclear, the regularized process has been associated with reduced signal transmission speed and coordination,41 which may be compensated by higher local efficiency to preserve efficient communication.42
Further analysis of regional and network measures revealed both increased and decreased patterns of nodal strength and iFC in women with bulimia nervosa. The increased nodal strength and iFC primarily involved sensorimotor and unimodal visual (mainly in the occipital cortex) regions. The sensorimotor cortex provides an obligatory portal for the entry of sensory information into the cortical circuitry and is important for awareness of body sensations, size and spatial positions,31 whereas the occipital cortex mediates visual perception of body shape and/or size.43 The sensorimotor and occipital regions are thus potentially relevant to the behavioural manifestation of distorted body image (i.e., overestimation of body size, body dissatisfaction and body weight control), which has an important role in the onset and maintenance of eating disorders44 and seems to be more pronounced in patients with bulimia nervosa than in those who have anorexia nervosa.45
The relevance of the sensorimotor and occipital regions to distorted body image has been emphasized in several previous studies.9–11,14 For instance, reduced occipitotemporal responses during presentations of underweight or overweight human bodies were observed in patients with bulimia nervosa;9 the lateral occipital activation was sensitive to stimuli of body size distortions in healthy people, whereas such a modulation was not observed in those with bulimia nervosa.10 At rest, patients with bulimia nervosa showed abnormal iFC within the somatosensory network and between the paracentral lobule and 2 occipital regions.14 In the present study, we provide evidence for bulimia nervosa–related dysfunctions in the sensorimotor and visual regions within a larger whole brain context. The strengthened coupling between sensorimotor and occipital regions may reflect a dysfunctional processing of sensorimotor information about perceived body size; such an anomalous multisensory integration might be also explained by the inclination of patients with bulimia nervosa to use somatosensory means to check and hide their body size (e.g., repeated weighing, wearing bulky clothes, etc.). The clinical implication of our findings is further strengthened by significant positive correlations observed between nodal strength in several unimodal visual regions and scores of bulimia and drive for thinness.
We also found increased nodal strength in the left precuneus and increased iFC between the precuneus and several sensorimotor and unimodal association regions. The precuneus, a core component within the default mode network, is involved in self-oriented processes,46 such as describing one’s physical appearance and personality trait.47 Preliminary evidence has indicated aberrant precuneus iFC with the somatosensory regions14 and dorsal ACC.15 We therefore speculate that altered precuneus connectivity may be a candidate mechanism for the core features of bulimia nervosa (i.e., overestimation of the importance of their bodily appearance instead of other aspects of performance and personality).48
Conversely, we found bulimia nervosa–related decreases in nodal strength in the OFC, medial temporal lobe (hippocampus and PHG) and subcortical regions (insula, amygdala, putamen and thalamus) and in iFC extending across subcortical (striatum, thalamus), limbic (amygdala, hippocampus) and paralimbic (ACC, PHG and OFC) systems, particularly for connectivity between subcortical regions and sensorimotor and unimodal visual regions. The striatum, thalamus, ACC, OFC, amygdala and hippocampus are associated with reward function,49 which has been disturbed in individuals with eating disorders.50 As reported previously, patients with bulimia nervosa showed reduced neural responses to a task paradigm generating reward values across a large network covering the insula, striatum, amygdala and OFC;2 such a response was predicted by binge/purge frequency.2 The abnormal response to food versus nonfood images in reward regions has been shown to differentiate anorexia nervosa and bulimia nervosa.8 Based on these studies, our findings of decreased nodal strength in these reward-related regions and their iFC with sensorimotor and visual regions might indicate a reward hyposensitivity to disorder-specific signals, such as taste and bodily stimuli, in patients with bulimia nervosa. The downregulated reward system may drive the individuals to binge eating to compensate, followed by purging behaviours to prevent weight gain. Such a behavioural explanation could be further confirmed by our findings of significant negative correlations in iFC between several subcortical regions and sensorimotor and visual regions with bulimia scores of the EDI-1.
Given that the reward function involves a series of neural processes, it is of interest to speculate on more specific behavioural implications of our findings. The amygdala, striatum and OFC have been thought to mediate response to a reinforcing stimulus, reward learning and evaluation, respectively,51 whereas the ACC and PFC are responsible for reward-related decision-making.52 Preliminary evidence has implicated these regions in bulimia nervosa–related dysfunction in several reward-related behavioural domains. Specifically, patients with bulimia nervosa showed exaggerated response to food stimuli in the striatum and OFC5 and reduced amygdala response to disgusted facial expressions.4 Altered activity in the ACC and OFC has been observed in patients with bulimia nervosa during impulse-control tasks, which has been associated with self-regulatory deficits12,13 (i.e., an inability to inhibit impulses in favour of a delayed reward). Although it plays an important role in the processing of interoception-reward signals,53 disturbed insula response to sucrose taste has been implicated in interoceptive deficits (i.e., reduced ability to feel full or hungry) in individuals with bulimia nervosa.3,53 It could be speculated that patients with bulimia nervosa may be impaired in a series of reward-related processes, which contributes to an inability to accurately identify emotional significance, generate appropriate behaviours and make decisions concerning eating and bodily stimuli.22
Our findings also pointed to a less commonly considered region in patients with bulimia nervosa — the thalamus. The thalamus relays communication among subcortical and cortical regions and plays a central role in the integration of sensory information.54 A recent study has shown abnormal structural integrity in the thalamic radiation tract of patients with bulimia nervosa.55 Our study confirmed that the thalamus functional connectivity was also impaired, as reduced iFC between the thalamus and sensorimotor and unimodal visual regions and negative associations between the thalamus connectivity and BMI were found in patients with bulimia nervosa. The finding might suggest impaired transmission processes of sensory and visual signals. In addition, given the knowledge that the hippocampus and PHG are involved in visuospatial perception and contextual memory processes,56,57 we speculate that our findings of reduced nodal strength in the hippocampus and PHG and PHG–OFC connectivity may contribute to the coexisting impairment of visuospatial ability and visual memory in women with bulimia nervosa.58
Limitations
Strengths of our study include the large unmedicated sample of patients with acute bulimia nervosa who had a short duration of illness. However, several issues need to be further addressed. It is unclear whether the topological abnormalities comprise vulnerability markers for the disorder developing or whether they are the results of disorder-specific symptoms and behaviours. Studying patients after recovery will be necessary to disentangle these trait and state effects. Future studies might also examine the effects of cognitive behavioural or pharmacological therapies on neural processes in patients with bulimia nervosa. Such an intervention design may provide deeper insights into the neural mechanisms of the disorder and allow for the identification of predictors of treatment outcome. In addition, the disorder specificity of our findings remains to be clarified, given that the dysfunctions in some of the regions, such as the striatum, thalamus and OFC, have also been detected in patients with a range of other mental disorders.59–62 Finally, as this is, to our knowledge, the first study of topological organization of bulimia nervosa and as so far only a few resting-state fMRI studies have been conducted in patients with this disorder, the results and their interpretation are still tentative. A combined design of resting-state and task-based paradigms would be helpful for strengthening the understanding of the behavioural implications of our findings.
Conclusion
We found women with bulimia nervosa to have altered intrinsic functional brain architecture: specifically, altered global and local efficiency and nodal- and network-level connectivity involving the sensorimotor and visual, limbic, paralimbic and subcortical systems. These findings support the proposal that bulimia nervosa is a disorder of dysfunctional integration among large-scale, distributed brain regions/networks. The characteristic abnormalities may contribute to more comprehensive understanding of the behavioural complexities of bulimia nervosa and further help identify potential novel therapeutic targets for this disorder.
Acknowledgements
The authors are grateful for funding from the National Key Technology R&D Program (2015BAI13B01), Beijing Municipal Science and Technology Commission (D121100005012002 and Z161100000216152), Doctoral Program of Higher Education of China (20130001110106), National Key Basic Research Program of China (973 Program) (2013CB531305), National Natural Science Foundation of China (81671774), and the Hundred Talents Program of the Chinese Academy of Sciences.
Footnotes
↵* These authors contributed equally to this work.
Competing interests: C. Correll is on the boards of Alkermes, Intra-Cellular Therapies, Janssen, Johnson & Johnson, Lundbeck, Neurocrine, Otsuka, Pfizer, Sunovion and Teva; a consultant for Alkermes, Allergan, the Gerson Lehrman Group, IntraCellular Therapies, Janssen, Johnson & Johnson, LB Pharma, Lundbeck, Medscape, Otsuka, Pfizer, Sunovion, Takeda and Teva; has been paid by Bristol-Myers Squibb, Janssen, and Otsuka for expert testimony; has received speaker fees from Alkermes, Allergan, the Gerson Lehrman Group, IntraCellular Therapies, Janssen, Johnson & Johnson, LB Pharma, Lundbeck, Medscape, Otsuka, Pfizer, Sunovion, Takeda and Teva; and has received payment from Medscape for manuscript preparation. No other competing interests declared.
Contributors: Q. Kong, K. Li, X. Li, D. Zhang and T. Si designed the study. L.Wang, Y. Zeng, C. Chen, Y. Qian, S. Feng, J. Li and Y. Su acquired the data, which C. Correll, P. Mitchell and C. Yan analyzed. L. Wang wrote the article, which all authors reviewed and approved for publication.
- Received September 13, 2016.
- Revision received November 7, 2016.
- Revision received February 6, 2017.
- Accepted March 8, 2017.