Abstract
Background: It is unknown whether impaired coupling among 3 core large-scale brain networks (salience [SN], default mode [DMN] and executive control networks [ECN]) is associated with relapse behaviour in treated heroin-dependent patients.
Methods: We conducted a prospective resting-state functional MRI study comparing the functional connectivity strength among healthy controls and heroin-dependent men who had either relapsed or were in early remission. Men were considered to be either relapsed or in early remission based on urine drug screens during a 3-month follow-up period. We also examined how the coupling of large-scale networks correlated with relapse behaviour among heroin-dependent men.
Results: We included 20 controls and 50 heroin-dependent men (26 relapsed and 24 early remission) in our analyses. The relapsed men showed greater connectivity than the early remission and control groups between the dorsal anterior cingulate cortex (key node of the SN) and the dorsomedial prefrontal cortex (included in the DMN). The relapsed men and controls showed lower connectivity than the early remission group between the left dorsolateral prefrontal cortex (key node of the left ECN) and the dorsomedial prefrontal cortex. The percentage of positive urine drug screens positively correlated with the coupling between the dorsal anterior cingulate cortex and dorsomedial prefrontal cortex, but negatively correlated with the coupling between the left dorsolateral prefrontal cortex and dorsomedial prefrontal cortex.
Limitations We examined deficits in only 3 core networks leading to relapse behaviour. Other networks may also contribute to relapse.
Conclusion: Greater coupling between the SN and DMN and lower coupling between the left ECN and DMN is associated with relapse behaviour. These findings may shed light on the development of new treatments for heroin addiction.
Introduction
Cognitive dysfunction is a key component of addictive disorders. With heroin addiction, specific deficits in salience attribution and inhibitory control emerge during long-term drug abuse and promote heroin relapse during withdrawal.1 Although most heroin-dependent individuals are willing to quit, available interventions for heroin addiction have shown only limited efficacy so far. Methadone maintenance treatment programs (MMTPs) are a common treatment approach thought to be effective for heroin addiction; however, the heroin-dependent patients in MMTPs are at high risk for relapse, with about 70% of individuals relapsing once they leave such a program.2 Despite the high relapse rate, few neuroimaging studies so far have addressed the neural predictors of risk for heroin relapse. Studies using task-related functional MRI (fMRI) have shown some usefulness in identifying relapse predictors3–5 or quitting predictors.6,7 For example, a previous study by our group showed that drug-related cues induced activity of the nucleus accumbens/subcallosal cortex and that the cerebellum was associated with subsequent relapse in heroin-dependent individuals.3 Brain activity associated with error processing was shown to predict relapse in cocaine-dependent patients.4 Disrupted ventromedial prefrontal function was shown to be associated with relapse in alcohol-dependent patients.5 Greater response of the medial prefrontal cortex and precuneus to self-related tailored smoking-cessation messages was shown to be associated with quitting.6 And pretreatment medial prefrontal cortical response to heroin-related cues was shown to be associated with greater adherence to injectable extended-release naltrexone.7 However, more effective imaging markers that are highly predictive of treatment outcome are critical for developing new or improved therapeutic strategies.
There is growing evidence that drug addiction is associated with alterations in system-level interactions between brain networks rather than with a functional breakdown within only a single discrete region.8 Patterns of regional brain activity are reflected in oscillations in large-scale brain networks in the resting state.9 Recently, a triple brain network model was proposed, including the default mode network (DMN), salience network (SN) and executive control network (ECN).10 The ECN is involved in processing of attention and external stimuli, whereas the DMN is implicated in stimulus-independent processing, such as self-referential thinking. The SN facilitates allocating attention toward the internal or external stimuli. Studies have shown the anticor-related patterns between the DMN and ECN,11,12 and the SN plays a role in modulating relative activity in the DMN and ECN.13,14 The aberrant intrinsic organization and interconnectivity of the SN, ECN and DMN may underlie neuropsychiatric disorders, including addiction.10 It was recently proposed that during a nicotine-deprived state the SN of the smokers allocates enhanced attention resources to internal symptoms of withdrawal and biasing activity to the DMN and away from the ECN.15 Lerman and colleagues16 found that alterations in functional connectivity of the SN and DMN and the inability to disengage from the DMN may be critical in cognitive/affective alterations that underlie nicotine dependence. Also, studies of cocaine addiction revealed that the interaction between the SN and DMN was disrupted.17,18 However, the exact alterations of interactions between these 3 large-scale networks and their association with relapse behaviour in heroin-dependent individuals remain unknown.
In the present study, we recruited a cohort of heroin-dependent men participating in MMTPs and a cohort of healthy controls to investigate coupling among the large-scale brain networks. Given the triple network model and the evidence that the SN plays a role in toggling the resources between the DMN and ECN, we hypothesized that the functional connectivity between the SN and DMN would be stronger and that the functional connectivity between the SN and ECN would be weaker in heroin-dependent individuals who relapsed within 3 months after treatment in an MMTP than in those who remained abstinent and in the healthy controls. We also hypothesized that the functional connectivity between the SN and DMN and between the SN and ECN would be associated with the relapse behaviour.
Methods:
Participants
We recruited heroin-dependent men and healthy controls aged 22–49 years to participate in our study. Some of the participants with heroin-dependence had been included in our previous study.19 To be included in the present study, patients had to meet DSM-IV-TR7 criteria for heroin dependence, they had to have been receiving treatment in an MMTP for no less than 6 months, and they had to have been on a stable dose for at least 1 month before entering the study. All of the participants, including controls, were required to be free of DSM-IV-TR Axis I disorders or brain trauma and to be right-handed. The controls had to have no history of substance abuse except for nicotine. Other exclusion criteria were history of neurologic disease, current medical illness or recent medicine use, claustrophobia, and presence of magnetically active objects in the body. All participants gave written informed consent, and the ethics committee of Tangdu Hospital approved our study protocol.
Evaluation measures
We evaluated history of heroin use (lifetime use and use per day) and MMTP history (duration and dosage of methadone use). Basal heroin craving was assessed using a 0–10 visual analogue scale20–22 asking, “To what extent do you feel the urge to use heroin?” (0 indicated the least and 10 indicated the strongest craving).
Given clear evidence of the effectiveness of methadone for harm reduction in individuals with heroin dependence, MMTPs has been the most common treatment in China since 2004.23 Continuous MMTP treatment is encouraged for heroin-dependent patients; their long-term adherence to treatment relies on support from the MMTP clinic, family and society. Our procedures of longitudinal follow-up were in line with those described in a previous study.3 Briefly, heroin-dependent men were followed for 3 months. Follow-up sessions occurred at 1, 2 and 3 months after the experimental session. Our coordinator contacted the heroin-dependent men 3 days before each appointment and encouraged them to continue the MMTP. During each appointment, the patient underwent a structured interview assessing substance use and a urine drug screen. As opiates (mainly heroin, 38.1%) and synthetic drugs (mainly methamphetamine, 60.5%) are the main drugs of abuse in China,24 relapse was defined as any use of heroin or methamphetamine identified by positive urine drug screen (Morphine/Methamphetamine Diagnostic Kit, Guangzhou Jianlun Biological Technology co., LTD). Participants who missed the appointments without any contact with the study coordinator were considered to be relapsed. One patient dropped out during the second month of follow-up, but he had been identified as relapsed during the first month of follow-up. Other participants were considered to be in early remission.25
Image acquisition
No use of alcohol, tea, caffeine, or any drug or medicine was permitted in the 12 hours before the MRI scan. Scanning took place on the 3.0 T GE Signa Excite HD scanner at Tangdu Hospital. Prior to formal fMRI scan, participants underwent a mock scan for 1 minute to become familiar with the scanning environment. The formal scan began with a 10-second dummy scan followed by the data acquisition. During the formal scan, each participant was instructed to keep still, rest with their eyes passively viewing the white crosshair with black background projected in the centre of the mirror mounted on the head coil and not to think about anything special. The functional images were collected using a gradient echo planar imaging sequence with the following parameters: repetition time (TR) 2000 ms, echo time (TE) 30 ms, field of view (FOV) 256 × 256 mm2, imaging matrix 64 × 64, slice thickness 4 mm, gap 0 mm, 32 slices, flip angle 90°, spatial resolution 4 × 4 × 4 mm3. For each participant, 150 echoplanar volumes were collected during the resting-state fMRI scan. The acquisition duration of functional images lasted for 5 minutes. The corresponding high-resolution 3-dimensional T1-weighted images were also collected for spatial normalization of the data sets to a standard atlas. The fast spoiled gradient echo sequence was used: TR 7.8 ms, TE 3.0 ms, FOV 256 × 256 mm2, imaging matrix 256 × 256, slice thickness 1 mm, 166 slices, spatial resolution 1 × 1 × 1 mm3. The acquisition duration of structural images lasted for 7 minutes. The structural data were checked by an experienced radiologist (Q.L.) to identify structural abnormalities.
Imaging data procession
Data preprocessing using SPM 8 software (http://www.fil.ion.ucl.ac.uk/spm) and DPABI (http://rfmri.org/dpabi) included slice timing, motion correction, registration, spatial normalization to Montreal Neurological Institute (MNI) space with a resampled resolution of 3 × 3 × 3 mm3, and spatial smoothing (full width at half maximum 6 mm). The signals of white matter, cerebrospinal fluid and head motion parameters were removed through linear regression. Because excessive head motion can induce spurious correlation results in resting connectivity analysis, we used several steps to minimize the influence of head motion, such as excluding participants with head motion of more than 2 mm of translation and 2° of rotation in any of the x, y and z axes, scrubbing signal spikes based on the method of Power and colleagues,26 and including the head motion parameters as covariates.
Functional connectivity analyses
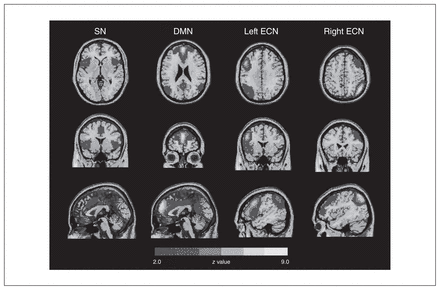
To define the large-scale brain networks, we performed a group independent component analysis using GIFT (http://mialab.mrn.org/software/gift/). The preprocessed data were submitted to GIFT with the component number set at 20 (14) and the algorithm set as Infomax. The group independent components spatial maps were converted into z score maps and thresholded to identify voxels contributing to each independent component. The SN, bilateral ECN and DMN were identified by visual inspection of the thresholded components maps (z > 2.0, corresponding to p < 0.05, 2-tailed;27 Fig. 1).
The large-scale networks derived from group independent component analyses. We identified 3 networks: the salience network (SN), default mode network (DMN), and the bilateral executive control network (ECN). Spatial maps for each brain network were converted to z score images, then thresholded at z > 2.0 (corresponding to p < 0.05, 2-tailed) and are showed in axial, coronal and sagittal views. The right side of the image corresponds to the right hemisphere of the brain. The key SN regions included the dorsal anterior cingulate cortex and bilateral anterior insula. The key DMN regions included the medial (ventromedial and dorsomedial) prefrontal cortex and posterior cingulate cortex. Together, the left and right ECN regions mainly included the bilateral dorsolateral prefrontal cortices and parietal cortices.
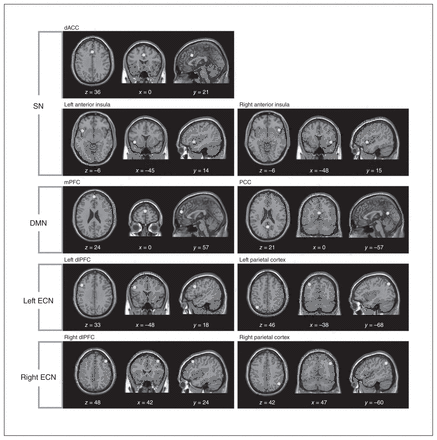
To investigate the concrete network coupling among the SN, bilateral ECN and DMN, we chose the peak voxel of each key region within the identified large-scale networks as centres of sphere-shaped regions of interest (ROI; radius 6 mm).28 Based on the group independent component analysis, 9 ROIs were defined, including 3 ROIs in the SN (dorsal anterior cingulate cortex [dACC] and bilateral anterior insula), 2 in the DMN (medial prefrontal cortex [mPFC] and posterior cingulate cortex [PCC]), 2 in the left ECN (left dorsolateral prefrontal cortex [dlPFC] and left parietal cortex) and 2 in the right ECN (right dlPFC and right parietal cortex; Fig. 2). The time courses for resting-state data were linearly detrended and temporally bandpass filtered (0.01–0.1 Hz). To restrict our analyses to the SN, bilateral ECN and DMN, we combined all of these identified spatial maps of large-scale brain networks as a mask. The voxel-wise functional connectivity analyses were performed within these networks based on the selected ROIs.
Nine regions of interest (ROI) were included in the different large-scale brain networks. The sphere-shaped ROIs (radius 6 mm) are displayed in Montreal Neurological Institute space. dACC = dorsal anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; DMN = default mode network; ECN = executive control network; mPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; SN = salience network.
The results of resting-state connectivity for each ROI were submitted to analysis of variance (ANOVA). The variation of cigarettes per day was controlled in the ANOVAs, and the variation of daily methadone dose was controlled when conducting post hoc analysis between the relapse and early remission groups. To account for testing 9 different ROIs, the critical cluster-corrected p value was set at 0.05÷9 = 0.006 based on Monte Carlo simulations.29 This threshold corresponded to a single-voxel uncorrected significance of p < 0.001 and a minimum cluster size of 270 mm3. For the heroin-dependent patients, correlations between percentage of positive urine drug screens, basal craving for heroin, duration and dose of MMTP and the coupling between each 2 large-scale brain networks showing ANOVA differences were examined and corrected for multiple comparisons using a method of false-discovery rate (FDR). To minimize the influence of methadone dose, we entered daily methadone dose as a covariate when conducting the correlation analysis.
Results:
Demographics and behaviour of study participants
We enrolled 73 participants in our study: 53 men with heroin dependence and 20 healthy controls. After the MRI scan, data from 3 patients with heroin dependence were discarded owing to excessive head motion, leaving a final sample of 50 patients and 20 controls. Twenty-six patients relapsed within the 3-month follow-up and 24 patients remained in early remission. Among those who relapsed, 10 patients had positive urine drug screens at the 1-month follow-up, 9 at the 2-month follow-up and 7 at the 3-month follow-up. Men in the relapse group reported a mean number of 1.4 ± 0.6 relapses and a mean dose of 0.6 ± 0.3 g of heroin used during the 3-month follow-up. None reported using methamphetamine. There were no significant differences in age, years of education and cigarettes smoked per day between the relapsed, early remission and control groups. The 2 heroin-dependent groups did not differ in basal heroin craving, lifetime heroin use, duration of MMTP, dosage of MMTP, or cigarettes smoked per day (Table 1).
Demographic and clinical characteristics of participants
Network connectivity analysis
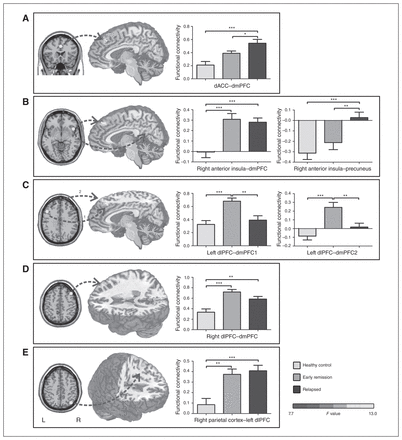
The relapsed group showed significantly greater functional connectivity between the dACC (included in SN) and dmPFC (included in DMN) than the early remission and control groups (p < 0.006, corrected using Monte Carlo simulations). In terms of functional connectivity based on the right anterior insula (included in SN), the relapsed and early remission groups consistently showed significantly higher functional connectivity with the dmPFC (included in DMN) than the control group (p < 0.006, corrected using Monte Carlo simulations). Meanwhile, the relapsed group showed significantly higher functional connectivity between the right anterior insula and precuneus (included in DMN) than the early remission and control groups (p < 0.006, corrected using Monte Carlo simulations; Fig. 3A and B and Table 2).
Differences in functional connectivity among the relapsed, early remission and healthy control groups based on analysis of variance of the regions of interest (ROIs) of the large-scale brain networks. The bar graphs represent values extracted from the entire differential clusters. dACC = dorsal anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; L = left; PCC = posterior cingulate cortex; R = right. *p < 0.05, **p < 0.01, ***p < 0.001. The p values are not corrected for multiple comparisons.
Differences in functional connectivity based on the ROIs of the large-scale brain networks
The relapsed and control groups showed significantly lower functional connectivity than the early remission group between the left dlPFC (included in left ECN) and 2 clusters of the dmPFC (included in DMN; dmPFC1, peak MNI coordinates x, y, z = 0, 30, 48; dmPFC2, peak MNI coordinates x, y, z = 24, 45, 42, p < 0.006, corrected using Monte Carlo simulations). Meanwhile, the relapsed and early remission groups consistently showed significantly higher functional connectivity between the right dlPFC (included in right ECN) and dmPFC (included in DMN) than the control group (p < 0.006, corrected using Monte Carlo simulations). In terms of functional connectivity based on the right parietal cortex (included in right ECN), the relapsed and early remission groups consistently showed significantly higher functional connectivity with the left dlPFC (included in left ECN) than the control group (p < 0.006, corrected using Monte Carlo simulations; Fig. 3C, D and E and Table 2).
No significant difference in functional connectivity based on the ROIs of the DMN (mPFC and PCC) survived the multiple comparison correction among the 3 groups. No significantly higher functional connectivity based on the 9 ROIs was found for the control group compared with the relapsed and early remission groups.
Correlation
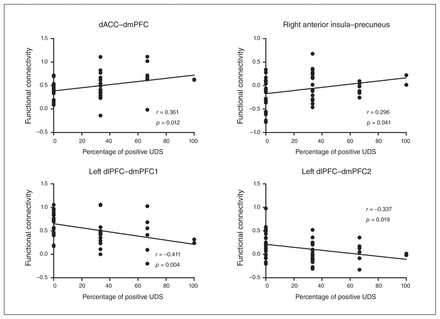
Because 1 participant with heroin dependence dropped out during the 3-month follow-up and there was no way to know his true percentage of positive urine drug screens, we excluded him from the correlation analysis. Across the patients with heroin dependence, the functional connectivity between the dACC and dmPFC positively correlated with the percentage of positive urine drug screens during the 3-month follow-up (r = 0.361, puncorrected = 0.012, pcorrected < 0.05). Also, we observed a trend of positive correlation between the right anterior insula–precuneus connectivity and the percentage of positive urine drug screens (r = 0.296, puncorrected = 0.041, pcorrected > 0.05), whereas the functional connectivity between the left dlPFC, dmPFC1 and dmPFC2 negatively correlated with the percentage of positive urine drug screens (r = −0.411, puncorrected = 0.004, pcorrected < 0.05 and r = −0.337, puncorrected = 0.019, pcorrected < 0.05; Fig. 4). We found no significant correlations between basal craving for heroin, duration and treatment dose at the MMTP and the coupling between large-scale brain networks that showed differences on ANOVA.
Association between coupling among 3 large-scale networks and relapse behaviour. Correlation maps between percentage of positive urine drug screens (UDS) and functional connectivity strength of selected regions of interest in patients with heroin dependence. The p values shown are not corrected for multiple comparisons. dACC = dorsal anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex.
Discussion
To our knowledge, this is the first resting-state neuroimaging study to assess coupling of large-scale networks associated with relapse in men with heroin addiction. Our findings showed that abnormally higher functional connectivity between the SN and DMN and lower functional connectivity between the left ECN and DMN were associated with relapse behaviour in treated patients with heroin dependence. No difference in the coupling of the SN and ECN between the relapse and early remission groups was found. These results suggest that stronger SN–DMN coupling and weaker left ECN–DMN coupling may be the driving force behind the relapse behaviour. More generally, our findings also contribute to a growing body of literature in which neuroimaging measures are used to evaluate the outcome of therapies for heroin addiction.7,30–34
The SN plays a role in salience detection and attention capture induced by error signals and dynamic cognitive control.14 Increasing evidence suggests that the SN is the hub in mediating dynamic interactions between other large-scale networks, such as the ECN (involved in externally oriented attention) and the DMN (involved in internally oriented self-related mental processes).10 As 3 nodes of the SN, the dACC and bilateral anterior insula are part of a functional circuit implicated in attention, interoceptive and affective processes.14 It has also been suggested that this network plays a key function in identifying the most homeostatically relevant among several internal and external stimuli to guide behaviour.13,35,36 On the other hand, Naqvi and colleagues37 found that smokers with brain damage involving the insula were more likely to quit smoking. The precuneus and dmPFC are key regions of the DMN38,39 that have been found to be impaired in heroin users.3,39,40 The precuneus is involved mainly in processing self-reflection and self-awareness.41 The dmPFC has been associated with emotional appraisal.7 Our findings of higher functional connectivity between the SN (dACC and right anterior insula) and DMN (dmPFC and precuneus) in the heroin-dependent groups than in the control group might suggest that the patients with heroin dependence are characterized by abnormally higher mental processes of internally oriented salience attribution. Patients with heroin dependence who have strong SN–DMN functional connectivity may experience difficulties in disengaging from self-focused thoughts related to drug cue, negative withdrawal symptoms or stress, as supported by the positive association between the dACC–dmPFC connectivity and the trend toward a positive association between right anterior insula–precuneus connectivity and relapse behaviour. These patients are vulnerable to relapse; a possible interpretation is that their SN increases the allocation of attentional resources to attend to drug cues, withdrawal symptoms or stress-induced urges, leading to relapse behaviours.
Meanwhile, we found that the relapsed group showed significantly lower functional connectivity between the left ECN (left dlPFC) and DMN (dmPFC) than the early remission group. We also found a trend of lower right ECN (right dlPFC)–DMN (dmPFC) connectivity in the relapsed group than in the early remission group, although this difference was not statistically significant. The ECN is crucial for decision-making in the context of goal-directed behaviours, for rule-based problem-solving and for actively manipulating and maintaining information in working memory.42–45 As a key node of the ECN, the dlPFC has been predominantly involved in top–down inhibitory control, emotion regulation, and decision-making.46,47 The dmPFC has also been associated with the evaluative and decision-making aspects of self-related processing.6,7,48 Successful quitting requires top–down executive cognitive control over urges to use heroin. The weaker left ECN–DMN functional connectivity in the relapsed group than in the early remission group together with the negative association between the left dlPFC–dmPFC connectivity and relapse behaviour, our data might suggest that patients with heroin dependence who have weak ECN–DMN functional connectivity may also experience difficulties in engaging inhibitory control over self-focused thoughts related to drug cues, withdrawal symptoms or stress-induced urges. These patients are also vulnerable to relapse; a possible interpretation is that the ECN allocates less resources of inhibitory control over drug cue, withdrawal symptom or stress-induced urge, leading to relapse behaviours.
However, we also found significantly higher functional connectivity between the bilateral ECN (bilateral dlPFC) and DMN (dmPFC) in the early remission group than in the control group. As the patients in early remission tend to remain in long-term, stable MMTPs focused on relapse prevention, a possible explanation is that MMTPs help to build a new balance of inhibitory control circuits based on the neuroplastic adaptations resulting from long-term methadone use. A variety of studies have shown the role of methadone in reducing heroin craving, alleviating withdrawal symptoms and, in turn, decreasing relapse rates.49 The patients in early remission may benefit from the MMTP, and their ECN may be able to exert more inhibitory strength over the DMN than it does in healthy controls. Nevertheless, future longitudinal studies on the exact role of methadone in modulating inhibitory control in a larger cohort of patients with heroin dependence are warranted.
Contrary to our hypothesis, we did not find weaker SN–ECN connectivity in the relapsed group than in the early remission group. A possible explanation might be that the difference in SN–ECN coupling between relapsed and early remission groups was too subtle to detect in a small sample. Second, our methods showed only bilateral functional connectivity between different networks, so it is still unknown whether there is significant difference in effective functional connectivity between the relapsed and early remission groups. Future work is needed to clarify exact characteristics of coupling between the ECN and SN based on a larger cohort of patients with heroin dependence and more sophisticated methods, such as Granger causality analysis50 and dynamic causal modelling.30,31
Although purely speculative, lowering the strength of connectivity between the SN and DMN and elevating the strength of connectivity between the ECN and DMN, which increase the ability of inhibition between the ECN and DMN and between the SN and DMN, may attenuate relapse behaviours and could thus have potential treatment implications (e.g., use of noninvasive brain stimulation methods, such as transcranial magnetic stimulation, and/or mindfulness therapeutics). Therefore, exploration of the association between the functional connectivity of large-scale networks and relapse behaviour in a longitudinal cohort study would be important.
Limitations
Despite the strengths of this study, our results should be interpreted in light of potential limitations. First, this study was based on a hypothesized model of deficits in triple network interactions leading to relapse behaviour; we did not explore whether other network components are associated with relapse behaviour. Other networks may also contribute to the relapse mechanism. Second, owing to the relatively long interval between each time points of follow-up, we could not determine the exact number of relapses in the heroin-dependent group. Therefore, we chose the objective results of urine drug screens at each follow-up assessment as an indicator of relapse behaviour. Third, although the patients with heroin dependence were not directly warned about a possible urine drug screen 3 days before the follow-up visit, inevitably, some of them were more or less motivated or able to conceal their intermittent heroin use. Therefore, random urine drug testing may be a better approach during the follow-up. Fourth, owing to limited availability of female patients and to the difficulty of data collection in our district, we restricted our sample to include only men. Therefore, it is not known whether our findings could be generalized to women with heroin dependence, and this requires further study. Finally, owing to the very limited cocaine and cannabis dependence in China24 and strict control on prescription of opioids, such as methadone and buprenorphine, we did not test for these drugs in the urine drug screen.
Conclusion:
Our data go beyond a single dysregulated brain region/network and show that strong functional connectivity between the SN and DMN and weak functional connectivity between the left ECN and DMN is associated with relapse behaviour in patients with heroin dependence. These findings advance our understanding of neural mechanisms of heroin addiction and reveal deficits in network interactions that may increase relapse risk. Our findings may therefore shed light on the development of treatment targets for preventing relapse in individuals with heroin addiction.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81671661, 81201081, 81371532 and 81401393) and the Technology Innovation Development Foundation of Tangdu Hospital (No. 2013LCYJ003). The funders had no role in the design and conduct of the study; data collection, analysis, or interpretation of the data; preparation and approval of the manuscript; or the decision to submit the manuscript for publication. The authors thank Mrs. Yan Meng (Department of culture and media, Shaanxi Youth Vocational College) for editing the figures and Mr. Liyan Zhao (National Institute on Drug Dependence, Peking University) and Mr. Yonggui Yuan (Department of Psychosomatics and Psychiatry, Zhongda Hospital, School of Medicine, Southeast University) for improvement of this study.
Footnotes
↵* These authors contributed equally to this work.
Competing interests: None declared.
Contributors: Q. Li and W. Wang designed the study. J. Liu, Y. Wang, W. Li, J. Chen, J. Zhu, X. Yan, Y. Li, Z. Li and J. Ye acquired the data, which Q. Li and J. Liu analyzed. Q. Li, J. Liu and W. Wang wrote the article, which all authors reviewed and approved. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received January 12, 2017.
- Revision received May 9, 2017.
- Revision received June 20, 2017.
- Accepted June 22, 2017.