Abstract
Background: The nucleus accumbens (NAcc) has been implicated in the pathology and treatment of schizophrenia. Recent postmortem evidence suggests a hyperglutamatergic state in the NAcc. With the present study we aimed to explore possible glutamatergic alterations in the NAcc of a large schizophrenia cohort.
Methods: We performed immunoblots on postmortem NAcc samples from 30 individuals who had schizophrenia and 30 matched controls. We examined the protein expression of primary glutamatergic receptors, including the N-methyl-d-aspartate (NMDA) receptor (NR1, NR2A and NR2B subunits) and the group 1 metabotropic glutamate receptor (mGluR1 and mGluR5; dimeric and monomeric forms). In addition, we measured the group 1 mGluR endogenous regulators, neurochondrin and Homer1b/c, which have recently been implicated in the pathophysiology of schizophrenia.
Results: Protein levels of glutamatergic receptors and endogenous regulators were not significantly different between the controls and individuals who had schizophrenia. Furthermore, mGluR5, but not mGluR1, showed a positive association with NMDA receptor subunits, suggesting differential interactions between these receptors in this brain region.
Limitations: Investigation of these proteins in antipsychotic-naive individuals, in addition to the subregions of the NAcc and subcellular fractions, will strengthen future studies.
Conclusion: The present study does not provide evidence for glutamatergic abnormalities within the NAcc of individuals with schizophrenia. Taken together with the results of previous studies, these findings suggest NMDA receptors and group 1 mGluRs are altered in a brain region–dependent manner in individuals with schizophrenia. The differential associations between mGluR1, mGluR5 and NMDA receptors observed in this study warrant further research into the interactions of these proteins and the implications for the therapeutic and adverse effect profile of glutamatergic-based novel therapeutics.
Introduction
The nucleus accumbens (NAcc), a major component of the ventral striatum, is believed to play a large role in the pathology of schizophrenia; however, there has been little direct investigation of this region in individuals with schizophrenia. Current antipsychotics are thought to target the NAcc and reduce a striatal hyperdopaminergic state, which was implicated in schizophrenia by early imaging and pharmacological studies.1,2 However, recent and further advanced imaging studies capable of analyzing the subregions of the striatum suggested the NAcc may not show a hyperdopaminergic state as much as neighbouring striatal regions.3,4 In agreement with this idea, a recent postmortem study reported unaltered protein levels of the rate-limiting dopamine synthesis enzyme tyrosine hydroxylase within the NAcc.5 However, protein expression of the vesicle glutamate transporter VGLUT2 was found to be increased, suggesting altered glutamatergic signalling within the NAcc.6 In support of this finding, increased asymmetric synapses, representative of glutamatergic projections, have been observed within the core of the NAcc in individuals with schizophrenia.7 Furthermore, these synapses were observed to have reduced postsynaptic density area. Taken together, these recent studies suggest that altered glutamatergic neurotransmission to the NAcc is associated with schizophrenia pathology; however, further evidence characterizing the glutamatergic system within the NAcc in individuals with schizophrenia is required.
The NAcc receives dense excitatory input from the thalamus, hippocampus, prefrontal cortex (PFC) and amygdala, regions strongly associated with glutamatergic alterations in schizophrenia pathology.8 Alterations of N-methyl-d-aspartate (NMDA) receptor expression within the PFC, hippocampus and amygdala, have been reported previously.9 Although early studies investigating the NMDA receptor in the NAcc within postmortem tissue from individuals who had schizophrenia reported no changes in binding or subunit messenger RNA (mRNA) levels,10–12 these studies were limited by small cohorts (n = 8–15/group) with no examination at the protein level. The NMDA receptors colocalize with group 1 metabotropic glutamate receptors (mGluR), including mGluR1 and mGluR5. Activation of NMDA receptors can regulate group 1 mGluR signalling.13 Furthermore, activation of group 1 mGluRs in striatal neurons, in particular mGluR5, potentiates NMDA receptor activity.14 Therefore, NMDA receptors and group 1 mGluRs may be altered in a concerted manner in individuals with schizophrenia. A previous study reported no change of group 1 mGluR protein expression in the NAcc in individuals with schizophrenia (9 controls, 16 cases).15 However, it is important to note that there was no measure of the dimeric or monomeric forms of the mGluRs, which are now known to regulate the functional properties of these receptors.16 We recently identified that protein expression of group 1 mGluRs, particularly the dimeric form, was altered in 2 regions that innervate the NAcc and that were associated with the glutamatergic dysfunction of schizophrenia: the PFC and hippocampus.17–19 Therefore, considering these regions send major glutamatergic projections to the NAcc and the recent evidence this system may be disrupted in individuals with schizophrenia, we hypothesized that these proteins may be altered in the NAcc of individuals with schizophrenia.
Group 1 mGluR downstream signalling and cellular localization is positively mediated via several endogenous regulators, including neurochondrin (referred to as Norbin in rodents) and Homer1b/c.20 Similar to mGluR1/5 knockout (KO) mice,21–23 KO models of Norbin and Homer1 show schizophrenia-like behaviours, such as prepulse inhibition deficits and increased hyperlocomotor response following the administration of the NMDA receptor antagonist MK-801.24,25 In line with this finding, genetic studies show an association between Homer1 polymorphisms and Positive and Negative Syndrome Scale (PANSS) scores as well as therapeutic response.26 Furthermore, we and others have reported alterations of neurochondrin and Homer1b/c mRNA and protein expression in the hippocampus and cortex in postmortem schizophrenia cohorts, several of whom displayed concurrent group 1 mGluR alterations.17–19,27
With the present study we aimed to further investigate if glutamatergic alterations exist in the NAcc of individuals with schizophrenia and examine, to our knowledge, for the first time whether protein expression of the NMDA receptor complex and dimerization of group 1 mGluRs were altered in individuals who had schizophrenia. To further explore potential glutamatergic dysregulation in the NAcc in schizophrenia, we assessed the protein expression of the group 1 mGluR endogenous regulators neurochondrin and Homer1b/c in a large postmortem schizophrenia cohort.
Methods
Human postmortem samples
Human postmortem NAcc tissue was obtained from the New South Wales Brain Tissue Resource Centre (Sydney, NSW, Australia). The cohort consisted of 30 individuals who had schizophrenia (including 7 who had schizoaffective disorder) and 30 controls, which to our knowledge represents the largest postmortem NAcc cohort in schizophrenia research. Schizophrenia/schizoaffective disorder was diagnosed according to the DSM-IV through review of medical records by experienced clinicians using the Diagnostic Instrument for Brain Studies (DIBS), a postmortem clinical assessment tool, as previously detailed.28 Controls had no history of psychiatric illness or drug abuse and were matched to the schizophrenia sample according to age at death, postmortem interval (PMI) and brain pH. There was no significant difference in age, freezer storage time, PMI or brain pH between the schizophrenia and control groups (Table 1). Premortem antipsychotic treatment was standardized to daily lifetime chlorpromazine equivalent (mg) and lifetime chlorpromazine equivalent (g) doses for each patient, and antidepressant treatment history was specified to a nominal scale (yes/no). Further demographic and clinical details regarding the cohort are outlined in Table 1 (see Appendix 1, Table S1, available at jpn.ca/170077-a1, for subdiagnostic demographic details). This study was approved by the University of Wollongong Human Research Ethics Committee (HE13/069).
Characteristics of the postmortem cohorts
Human brain tissue preparation
In brief, 20 mg of frozen NAcc from each control and each individual with schizophrenia was homogenized in 300 μL of buffer containing 0.1 M Tris-HCl, 2 mM EDTA, 10% glycerol, 1% SDS, 100 mM iodoacetamide, 0.5 mM phenylmethylsulfonyl fluoride, Protease Inhibitor Cocktail (P8340; Sigma) and Phosphatase Inhibitor Cocktail 2 (Sigma) and stored at −80°C. Total protein concentration was determined using a DC protein assay kit as per the manufacturer’s instructions (Bio-Rad).
Immunoblotting
We determined relative protein levels using Western blot, as previously performed by our group with minor modifications.29 In brief, samples (nonheated) were loaded at 10 μg and separated in 4%–20% TGX precast gels (Bio-Rad) under nonreducing conditions. This loading concentration was determined to be within the linear range of detection of all primary antibodies (Appendix 1, Fig. S1). Proteins were subsequently transferred onto polyvinylidene difluoride membranes (Bio-Rad). Membranes were probed with the following primary antibodies: anti-NR1 (1:5000; MAB363, Millipore), anti-NR2A (1:5000; MAB5530, Millipore), anti-NR2B (1:5000; MAB5578, Millipore), anti-mGluR1a (hereby referred to as mGluR1; 1:15 000; D5H10, Cell Signaling), anti-mGluR5 (1:5000; Ab27190, Abcam), anti-neurochondrin (Norbin; 1:7500; ab130507, Abcam) and anti-Homer1b/c (1:7500; ab211415 Abcam). Membranes were subsequently incubated with horseradish peroxidase conjugated secondary antibodies (1:5000; AP307P, Millipore, or 1:5000; AP308P, Millipore). Bands were visualized using Amersham ECL Western blotting detection reagent (GE Healthcare) and membranes exposed to Hyperfilm (GE Healthcare). Films were scanned using a GS-800 scanner (Bio-Rad), and densitometry values were quantified. Relative densitometry values for each protein were normalized to their respective glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels and an internal control sample (consisting of equal amounts of sample from each control and individual with schizophrenia pooled together) to account for protein loading and gel–gel variability, respectively. Each sample was run in duplicate or triplicate.
Statistical analysis
Statistical analyses were performed using SPSS software version 21. The level of significance was set to p < 0.05. We performed Kolmogorov–Smirnov tests to identify distribution of data. All protein measures showed normal distribution, with the exception of total, dimeric and monomeric mGluR1 and mGluR5, which were skewed to the right (Kolmogorov–Smirnov: 1.581 < Z > 2.430; < 0.001 < p > 0.014). Normal distribution of these protein measures were achieved by transforming to the natural logarithm of the relative protein values. We performed Pearson correlations to identify whether sample demographic characteristics (age, brain pH, PMI, freezer storage time) were associated with protein levels. Analyses of covariance (ANCOVA) examining diagnostic differences in protein measures were consequently carried out, covarying for factors significantly associated with protein levels. Where no covariates were identified, we performed analyses of variance (AVOVA) to examine differences in protein levels between the control and schizophrenia groups. We used 1-way ANOVAs to examine subdiagnostic (control, schizophrenia, schizoaffective disorder) differences of protein measures. We used 2-way ANOVAs to determine the effects of sex (male v. female) or hemisphere (left v. right) on each protein of interest as well as any interaction of these factors with diagnosis. We performed Pearson correlations to identify if age of disease onset, duration of illness and lifetime antipsychotic use (chlorpromazine equivalent) were associated with protein levels in schizophrenia samples, whereas we used independent t tests to assess whether antidepressant history (yes v. no) influenced measures of each protein of interest. Additional Pearson correlations were performed to determine the strength of association between protein measures.
Results
We clearly detected NMDA receptor subunits (NR1, NR2A and NR2B), group 1 mGluRs (dimeric and monomeric form), neurochondrin and Homer1b/c in the human NAcc in schizophrenia and control samples via immunoblotting (Fig. 1, Fig. 2 and Fig. 3). All proteins presented as a single band at their predicted molecular weights, with the exception of NR2A, mGluR1 and mGluR5. NR2A showed a strong band at 165 kDa and a fainter band at 145 kDa, which has previously been reported as a splice variant of NR2A using this antibody.30 The mGluR1 and mGluR5 dimers presented as 2 bands (270–280 kDa; quantified together as the dimer), whereas the monomer presented as a single band (150 kDa). We and others have previously reported this pattern of bands for group 1 mGluRs,29,31,32 including the use of alternate mGluR1 and mGluR5 antibodies, and specificity has been confirmed using respective KO mice.32,33 The mGluR1 dimeric and monomeric bands appeared at similar intensities. However, mGluR5 dimer bands were substantially more intense than mGluR5 monomeric bands (Appendix 1, Fig. S2), therefore, membranes were exposed for differing times to obtain clear and quantifiable bands for dimeric and monomeric mGluR5 separately. Total mGluR1 and mGluR5 levels were calculated by the sum of dimeric and monomeric values.
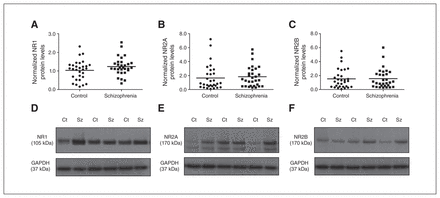
(A) NR1, (B) NR2A and (C) NR2B protein levels in the nucleus accumbens (NAcc) were unaltered between schizophrenia (squares) and control groups (circles). Representative immunoblots of N-methyl-d-aspartate (NMDA) receptor subunits (D) NR1 (105 kDa), (E) NR2A (165 kDa) and (F) NR2B (165 kDa) in individuals who had schizophrenia and controls. Representative glyceraldehyde 3-phosphate dehydrogenase (GAPDH) blots are shown below. Ct = control; Sz = schizophrenia.
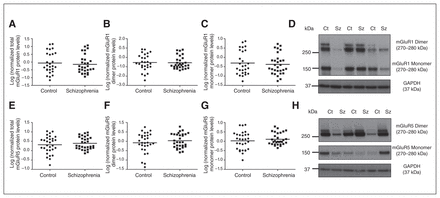
Total, dimeric and monomeric (A–C) mGluR1 and (E–G) mGluR5 expression was unaltered between control (circles) and schizophrenia (squares) groups. Representative immunoblots of dimeric (~270–280 kDa) and monomeric (150 kDa) (D) mGluR1 and (H) mGluR5 in controls and individuals who had schizophrenia. Representative glyceraldehyde 3-phosphate dehydrogenase (GAPDH) immunoblots are shown below. Ct = control; Sz = schizophrenia.
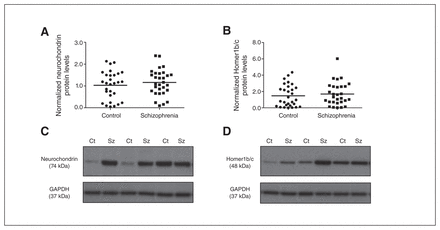
(A) Neurochondrin and (B) Homer1b/c protein levels were not significantly different in the nucleus accumbens (NAcc) between control (circles) and schizophrenia (squares) groups. Representative immunoblots of (C) neurochondrin (74 kDa) and (D) Homer1b/c (48 kDa) in controls and individuals who had schizophrenia. Representative glyceraldehyde 3-phosphate dehydrogenase (GAPDH) immunoblots are shown below. Ct = control; Sz = schizophrenia.
Diagnosis-related effects
Protein levels of all 3 NMDA receptor subunits did not differ significantly between the schizophrenia and control groups (NR1: F1,58 = 2.381, p = 0.13, covarying for PMI and freezer storage time; NR2A: F1,59 = 1.119, p = 0.50, covarying for brain pH; NR2B: F1,58 = 0.418, p = 0.52, covarying for age and brain pH; Fig. 1). In addition, we detected no influence of sex or hemisphere on NR1, NR2A and NR2B protein levels (all p > 0.05). Further quantification and statistical analysis of the NR2A splice variant (145 kDa band) and the cumulative values of the NR2A 165 and 145 kDa showed no significant difference between the control and schizophrenia groups (data not shown).
Total, dimeric and monomeric mGluR1 protein levels did not differ significantly between the control and schizophrenia groups (total: F1,59 = 0.744, p = 0.39, covarying for PMI; dimeric: F1,59 = 0.004, p = 0.95, covarying for freezer storage time; monomeric: F1,59 = 0.714, p = 0.40, covarying for PMI; Fig. 2). Similarly, total, dimeric and monomeric mGluR5 protein measures did not differ significantly between the control and schizophrenia groups (total: F1,60 = 0.563, p = 0.46; dimeric: F1,60 = 0.930, p = 0.34; monomeric: F1,60 = 0.617, p = 0.44). There was a significant main effect of hemisphere on total (F1,60 = 8.833, p = 0.004), dimeric (F1,60 = 7.783, p = 0.007) and monomeric (F1,60 = 10.115, p = 0.002) mGluR1 protein levels, which were significantly increased in the right hemisphere by 215%–216% compared with the left. There was, however, no interaction with diagnosis (p > 0.05). There was no effect of hemisphere or its interaction with diagnosis on mGluR5 protein levels. Furthermore, there was no significant influence of sex on any mGluR1 or mGluR5 protein measurements (all p > 0.05).
Group 1 mGluR endogenous regulators, Homer1b/c and neurochondrin, were not altered in the NAcc of the schizophrenia compared with the control group (neurochondrin: F1,59 = 0.807, p = 0.37, covarying for freezer storage time; Homer1b/c: F1,60 = 0.355, p = 0.55; Fig. 3). In addition, no effect of sex, hemisphere or interaction with these factors and diagnosis on Homer1b/c and neurochondrin protein levels were observed (all p > 0.05).
No significant differences were observed across all protein measures between the control (n = 30), schizophrenia (n = 23) and schizoaffective disorder (n = 7) groups (Appendix 1, Table S2).
Protein–protein correlations
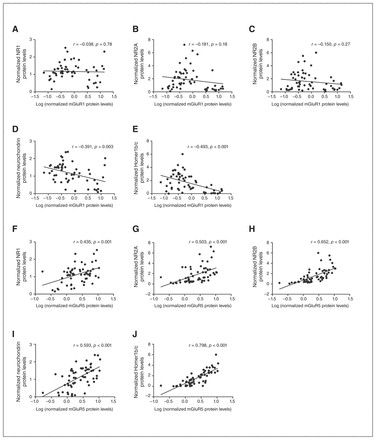
The mGluR5 protein levels were positively associated with NR1 (r = 0.435, p = 0.001), NR2A (r = 0.503, p < 0.001) and NR2B (r = 0.652, p < 0.001) protein levels (Fig. 4 and Appendix 1, Table S4). In contrast, mGluR1 was not associated with the NMDA receptor subunits (0.18 < p > 0.78). Furthermore, mGluR5 protein levels showed a strong positive correlation with neurochondrin (r > 0.500, p < 0.001) and Homer1b/c (r > 0.600, p < 0.001) in all controls and individuals who had schizophrenia, whereas a negative association was observed between mGluR1 and neurochondrin (r < −0.391, p = 0.003) as well as Homer1b/c (r < −0.493, p < 0.001) across all controls and individuals who had schizophrenia (Fig. 4 and Appendix 1, Table S5 and Table S6). For protein–protein associations within the control and schizophrenia groups, refer to Appendix 1, Table S5 and Table S6, respectively.
Correlation plots showed total mGluR1 protein levels did not correlate with N-methyl-d-aspartate (NMDA) receptor subunits (A–C) but negatively correlated with (D) neurochondrin and (E) Homer1b/c in all controls and individuals who had schizophrenia; however, total mGluR5 protein levels positively correlated with (F) NR1, (G) NR2A and (H) NR2B protein levels and (I) neurochondrin and (J) Homer1b/c.
Clinical characteristics
We observed significant positive associations between duration of illness and Homer1b/c (r = 0.448, p = 0.015) and NR2B (r = 0.465, p = 0.010). There were no significant associations observed between lifetime antipsychotic use and measured proteins (Appendix 1, Table S3). Furthermore, independent t tests showed none of the proteins of interest were influenced by antidepressant use (all p > 0.05). No other associations between the proteins of interest and clinical characteristics were observed (Appendix 1, Table S3).
Discussion
Glutamate is the main excitatory input onto the NAcc. Recent postmortem studies have suggested glutamatergic input to this region is increased.6,7 The NMDA receptor and group 1 mGluRs are primary glutamatergic receptors expressed on striatal neurons and are important for neuronal signalling and neurotransmitter tone. In the present study we show that protein expression of the NMDA receptor subunits (NR1, NR2A and NR2B), group 1 mGluRs (total, dimeric and monomeric forms) and group 1 mGluR regulators (Norbin and Homer1b/c) are not altered in the NAcc in individuals who had schizophrenia, which is in contrast to previous reports on cortical and hippocampal brain regions.9,17–19
In agreement with our findings, previous studies examining NMDA subunit transcript expression and receptor binding within the NAcc reported no change to NR1, NR2A and NR2B.11,12 Furthermore, no change in binding or mRNA expression of the other ionotropic glutamate receptors, amino-methylphosphonic acid and kainate, has previously been reported in the NAcc.12,34,35 The NMDA receptor shares a physical and functional link with the group 1 mGluRs. Despite the alterations of group 1 mGluR protein levels previously reported in the PFC, hippocampus and cerebellum, 17–19,36,37 here we report that in, to our knowledge, the largest NAcc postmortem schizophrenia cohort to date, group 1 mGluR dimer and monomer protein levels were unchanged between diagnostic groups. Similar to our findings, Gupta and colleagues15 reported no change in mGluR1 and mGluR5 protein expression in the NAcc of a small schizophrenia cohort of older adults; however, mGluR proteins were measured under reduced conditions unable to distinguish between the dimeric and monomeric forms. It is now clear that dimerization of mGluRs is critical for agonist-induced activation,16 therefore the present results add a deeper understanding. However, studies from the O’Malley laboratory have shown a large proportion of group 1 mGluRs are localized intracellularly, capable of activating different signalling pathways to their cell surface counterparts, highlighting the importance of examining specific cellular compartments.38,39 Although this was not feasible in the present study owing to tissue restrictions, we measured neurochondrin, an important regulator of mGluR5 cell surface trafficking.25 Consistent with our findings of no change to group 1 mGluR protein levels in the present study, we did not detect a difference in neurochondrin levels, which is in contrast to our previous findings in the PFC and hippocampus.17–19 However, it will be important for future studies to examine whether alterations occur in specific cellular compartments.
McCollum and colleagues7 reported an increase in asymmetric glutamatergic synapse number in the NAcc in a separate schizophrenia cohort, a finding that was specific to the core and not the shell of the NAcc, accompanied by a reduction in the area of the postsynaptic density (PSD) in asymmetric synapses. Furthermore, alterations of PSD membrane-associated guanylate kinases (MAGUK), SAP-102 and SAPAP1 expression have also been observed in the NAcc of schizophrenia cohorts, 40,41 suggesting alterations of scaffolding and signalling proteins within postsynaptic sites of the NAcc in schizophrenia pathology. The present study showed that this does not extend to Homer1b/c protein levels, one of the most abundant scaffolding proteins within the postsynaptic density, which functions as a multivalent adaptor complex for group 1 mGluRs.
Group 1 mGluRs and the NMDA receptor form physical and functional complexes. In line with this, we observed a positive correlation between mGluR5 and all NMDA receptor subunits investigated; however, this association was not present with mGluR1, suggesting a differential association between the NMDA receptor and group 1 mGluR subtypes in the human NAcc. In support of this, pharmacological studies have shown that the group 1 agonist 3,5-dihydroxyphenylglycine (3,5-DHPG) and specific mGluR5 agonist (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) are capable of potentiating NMDA receptor responses in striatal medium spiny neurons, an effect absent in mGluR5, but not mGluR1, KO mice.42 Furthermore, activation of striatal mGluR5, but not mGluR1, increases NMDA receptor cell surface expression, via CaMKIIa-mediated processes.43 These studies highlight the functional association between mGluR5, but not mGluR1, and the NMDA receptor in the striatum. With mGluR1- and mGluR5-based drugs in development for the treatment of psychiatric disorders, including schizophrenia, this association suggests that these compounds may have differential effects on NMDA receptor activity in the human NAcc. Further highlighting the differences between the group 1 mGluR subtypes, we observed mGluR5 was positively correlated, but mGluR1 was negatively associated, with the regulators Homer1b/c and neurochondrin. There is evidence to support this differential association, with transfection of Homer1c in mammalian cells increasing intracellular mGluR5 accumulation while having no effect on mGluR1.44 Other than the physical interaction of neurochondrin and mGluR1, to our knowledge, investigations of the functional interaction and signalling consequences of these proteins have yet to be conducted. Considerable research on neurochondrin has revolved around its interaction with mGluR5, whereby neurochondrin increases mGluR5 cell surface expression and downstream signalling and plays a role in mGluR5-dependent long-term potentiation.25
Limitations
There are several limitations of the present study that should be considered. McCollum and colleagues7 reported the increased density of characteristic glutamatergic synapses, in addition to a reduction in postsynaptic density. However, these changes were specific to the core and not the shell of the NAcc, suggesting subregional differences. Separate investigation of the core and shell in future studies may provide further insight on the pathology of these subregions in schizophrenia pathology. Furthermore, as previously discussed, group 1 mGluRs, in particular mGluR5, have various functions, dependent on their cellular localization.38,39 Future studies examining the expression of these proteins within subcellular compartments may provide a more in-depth understanding of possible functional pathologies.
A limitation of this study that must be considered is that individuals who had schizophrenia had a history of antipsychotic (n = 30) and antidepressant (n = 14) drug treatment. Antipsychotic and antidepressant drugs have little or no affinity for the glutamatergic system. Although, to our knowledge, there are no studies directly examining any possible effects of antidepressant drugs on glutamatergic proteins in the NAcc, the fact that we found no differences in these proteins between individuals with schizophrenia who had or had not been prescribed antidepressant drugs suggests this antemortem dosing did not influence our findings. In addition, we did not observe any associations between the glutamatergic receptors investigated in the present study and estimated lifetime antipsychotic use (measured as chlorpromazine equivalents). Further analysis of individuals with schizophrenia showed there were no differences in protein measures in the NAcc in those who had been prescribed predominantly typical antipsychotics (n = 19) compared with those who had been prescribed primarily atypical antipsychotics (n = 7; data not shown). In addition, our unpublished observations suggest that 10-week haloperidol treatment does not alter group 1 mGluR, Homer1b/c or Norbin protein expression in the NAcc. Our laboratory has previously shown that 10 weeks of haloperidol treatment can increase NR1 (but not NR2A) protein in the NAcc in a rodent model.45 In contrast, however, Fitzgerald and colleagues46 reported that 25 or 30 days of treatment with haloperidol or clozapine did not alter NR1 protein expression in the NAcc. Taken together, this suggests our findings in the NAcc of individuals who had schizophrenia are unlikely to be masked by the effects of antemortem drug treatment; however, it may be of interest to examine these proteins in a medication-naive cohort.
Conclusion
The present study found no change in protein expression of glutamatergic NMDA receptor subunits (NR1, NR2A, NR2B), dimeric and monomeric group 1 mGluRs and their endogenous regulators neurochondrin and Homer1b/c in the NAcc in a large postmortem cohort of individuals who had schizophrenia. Together with our previous findings in the PFC and hippocampus, this suggests these proteins are altered in a brain region–specific manner in individuals with schizophrenia. Although these results do not give credence to glutamatergic alterations within the NAcc, as recent studies suggest, this line of investigation deserves further examination.
Acknowledgments
This work was supported by the Schizophrenia Research Institute utilising infrastructure funding from the NSW Ministry of Health in the form of an A.M Woods Scholarship awarded to J.S. Lum. J.S. Lum is supported by Australian Rotary Health in the form of an Ian Scott Scholarship. This research has been conducted with the support of the Australian Government Research Training Program Scholarship awarded to J.S. Lum and S.J. Millard. Postmortem brain tissues were received from the NSW Tissue Resource Centre, which is supported by the National Health and Medical Research Council of Australia, Schizophrenia Research Institute and the National Institute of Alcohol Abuse and Alcoholism (NIH (NIAA) R24AA012725). This research was supported by the Schizophrenia Fellowship of NSW in the form of a Peter Meyer Fund Grant to K.A. Newell. L. Ooi is supported by a National Health and Medical Research Council (NHMRC) of Australia Fellowship (APP1135720).
- Received April 13, 2017.
- Revision received July 8, 2017.
- Accepted July 16, 2017.