Abstract
Background: Alterations in one-carbon metabolism have been associated with schizophrenia, and vitamin B6 is one of the key components in this pathway.
Methods: We first conducted a case–control study of serum pyridoxal levels and schizophrenia in a large Japanese cohort (n = 1276). Subsequently, we conducted a meta-analysis of association studies (n = 2125). Second, we investigated whether rs4654748, which was identified in a genome-wide association study as a vitamin B6-related single nucleotide polymorphism, was genetically implicated in patients with schizophrenia in the Japanese population (n = 10 689). Finally, we assessed the effect of serum pyridoxal levels on schizophrenia risk using a Mendelian randomization (MR) approach.
Results: Serum pyridoxal levels were significantly lower in patients with schizophrenia than in controls, not only in our cohort, but also in the pooled data set of the meta-analysis of association studies (standardized mean difference −0.48, 95% confidence interval [CI] −0.57 to −0.39, p = 9.8 × 10−24). We failed to find a significant association between rs4654748 and schizophrenia. Furthermore, an MR analysis failed to find a causal relationship between pyridoxal levels and schizophrenia risk (odds ratio 0.99, 95% CI 0.65–1.51, p = 0.96).
Limitations: Food consumption and medications may have affected serum pyridoxal levels in our cross-sectional study. Sample size, number of instrumental variables and substantial heterogeneity among patients with schizophrenia are limitations of an MR analysis.
Conclusion: We found decreased serum pyridoxal levels in patients with schizophrenia in this observational study. However, we failed to obtain data supporting a causal relationship between pyridoxal levels and schizophrenia risk using the MR approach.
Introduction
Schizophrenia is a devastating psychiatric disorder with a median lifetime prevalence of 0.7%–0.8%,1 and the etiopathogenesis of this disorder is still unknown.2 One-carbon metabolism is a process whereby folate transfers one-carbon groups in a range of biological processes, including DNA synthesis and methylation and homocysteine metabolism.3 Growing evidence indicates that alterations in one-carbon metabolism may play an important role in schizophrenia pathogenesis.4,5
To date, we have investigated potential associations between one-carbon metabolism factors and schizophrenia risk. For example, we observed elevated blood homocysteine levels in individuals with schizophrenia and provided evidence of a causal relationship between elevated blood homocysteine levels and schizophrenia risk.6,7 In addition, we showed that the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism, which causes reduced enzyme activity and elevated homocysteine levels, and common polygenic variants that are associated with plasma homocysteine levels are risk factors for schizophrenia in the Japanese population.6,8 Furthermore, we showed aberrant DNA methylation in patients with schizophrenia, not only in peripheral leukocytes but also in the postmortem dorsolateral prefrontal cortex.9–11
Vitamin B6 is a key component in one-carbon metabolism, in which homocysteine enters the transsulfuration pathway that leads to the production of cysteine and glutathione. The first enzyme in this pathway is cystathionine β-synthase, which catalyzes the conversion of serine and homocysteine to cystathionine and water, and requires vitamin B6 as a cofactor.5 To date, several cross-sectional studies have shown decreased serum pyridoxal levels (1 of the 3 forms of vitamin B6) in individuals with schizophrenia compared with controls.12–15 However, the sample size of these studies was small (total number of participants of each study ≤ n = 377), and a meta-analysis of association studies between serum pyridoxal levels and schizophrenia was not performed. Furthermore, it is still unclear whether pyridoxal levels are causally related to schizophrenia because of confounding factors and reverse causality in observational epidemiological studies.
In this study, we first investigated whether serum pyridoxal levels differed between patients with schizophrenia and nonpsychiatric controls in a large Japanese cohort (n = 1276). Subsequently, we conducted a meta-analysis of association studies between serum pyridoxal levels and schizophrenia (n = 2125). Second, we investigated whether rs4654748 in the neuroblastoma breakpoint family member 3 (NBPF3) gene, which was identified in a genome-wide association study (GWAS) as a vitamin B6–related single nucleotide polymorphism (SNP),3 was genetically implicated in schizophrenia in the Japanese population (n = 10 689). Finally, we assessed the effect of serum pyridoxal levels on schizophrenia risk using a Mendelian randomization (MR) approach, a useful tool for assessing causal associations in observational data.16 To show causality between serum pyridoxal levels and schizophrenia is important for understanding schizophrenia pathology and treatment, and an MR method, which utilizes genetic variants as instrumental variables for exposures of interest, can overcome problems in epidemiological studies, such as confounding and reverse causality.17
Methods
Participants in an association study of serum pyridoxal levels and schizophrenia
A total of 365 patients with schizophrenia (213 men, mean age 58.7 ± 9.5 yr; 152 women, mean age 59.6 ± 9.7 yr) were recruited from Tokushima University Hospital in Japan. Schizophrenia was diagnosed by at least 2 expert psychiatrists who used criteria listed in the DSM-IV based on extensive clinical interviews and a review of medical records. None of the patients exhibited any psychiatric comorbidity or cardiovascular disease. Most patients were being treated with various antipsychotic drugs. The median chlorpromazine equivalent dose was 550 mg/d (range 0 mg/d to 3450 mg/d). In addition, 911 control participants (297 men, mean age 38.7 ± 12.4 yr; 614 women, mean age 42.9 ± 12.0 yr) were selected from volunteers without structured interviews, who were recruited among hospital staff, students and company employees and who were documented to be free of psychiatric problems and without a history of mental illness. All study participants were of Japanese origin. The study protocol was approved by the institutional ethics committee of the University of Tokushima Graduate School, and all enrolled participants provided their written informed consent for participation. This experiment was performed in accordance with the Committee’s guidelines. Of the 1276 participants in this association study, 1224 provided genomic DNA (365 patients and 859 controls) for a genetic association study as described below.
Participants in a genetic association study between rs4654748 and schizophrenia
Four case–control sets were prepared from different facilities: the Tokushima University sample set (936 patients and 2440 controls), the Riken sample set (2004 patients and 2170 controls), the Osaka University sample set (451 patients and 602 controls), and the Fujita Health University sample set (1258 patients and 828 controls (Appendix 1, Table S1, available at jpn.ca/170053-a1). All study participants were of Japanese origin. The study protocol was approved by the institutional ethics committees of all participating institutes, and all enrolled participants provided written informed consent. All experiments were performed in accordance with the Committee’s guidelines. These sample sets have been described in previous studies.6,18–20
Serum pyridoxal measurements
Serum pyridoxal levels were measured using high-performance liquid chromatography (HPLC). We used an acid phosphatase to hydrolyze phosphate-ester compounds of pyridoxal. Next, we removed the protein using trichloroacetic acid. After adjusting the pH and using a reversed-phase column, we carried out the fractionation of pyridoxal and measured natural fluorescence with a spectrophotometer.
SNP genotyping
For genotyping, we used a commercially available TaqMan probe with an Applied Biosystems 7500 Fast Real-Time PCR System, according to the protocol recommended by the manufacturer (Applied Biosystems). The genotyping data of the Fujita Health University set was obtained from M. Ikeda (personal communication, Feb. 27, 2015), including previous GWAS data.19
Selection of studies for a meta-analysis of association studies between serum pyridoxal levels and schizophrenia
Eligible studies were identified in PubMed and ScienceDirect using the search terms vitamin B6 and schizophrenia. Studies were included for further meta-analysis if they met the following criteria: included laboratory assessment of serum pyridoxal levels, performed a case–control study (schizophrenia v. control), measured serum pyridoxal level using HPLC, and published in English. Two reviewers (M.K. and Y.T.) selected the articles independently according to the inclusion criteria. The scheme of the present study’s selection is shown in Appendix 1, Fig. S1.
Statistical analysis
Statistical analyses were performed with R software (version 3.2.3). Raw serum pyridoxal data were natural log-transformed before calculations, as performed in a previous study,21 because raw values of serum pyridoxal concentrations did not follow a Gaussian distribution. To validate the effect of rs4654748, which was identified in a previous GWAS,3 on serum pyridoxal levels in the Japanese control participants, we performed a multiple linear regression analysis, adjusting for age and sex. The β coefficient value, which represents a one-unit change in the natural log-transformed serum pyridoxal levels per copy increment in the C allele of rs4654748, was calculated as performed in our previous study.8 We performed a stratified analysis to examine the presence of differences in natural log-transformed serum pyridoxal levels between the 2 groups (schizophrenia v. control) separately by sex and by 3 genotypes of rs4654748 (total of 6 strata) after adjusting for age. Meta-analyses of association studies were performed on standardized mean differences (SMD), as performed in our previous study.6 In the meta-analysis of genetic association studies, we considered the C allele of rs4654748 as a reference allele. Heterogeneity was assessed using the I2 statistic. If heterogeneity across studies was found, then we applied a random-effects model; otherwise, we applied a fixed-effects model. We assessed publication bias using funnel plots and a regression test.22 We performed MR analysis using rs4654748 as the instrumental variable, as undertaken in our previous studies.6,7 An estimate for the C allele of rs4654748–serum pyridoxal level association was obtained from the present study of 859 Japanese control participants, as a β coefficient value (β pyridoxal/per allele). The risk estimate for an rs4654748–schizophrenia association was obtained from a current meta-analysis of the Japanese genetic association studies, as an odds ratio (OR) schizophrenia/per allele. An MR estimate for the effect of pyridoxal levels on schizophrenia risk was calculated, as an OR schizophrenia/pyridoxal. We used the following equation: log OR schizophrenia/pyridoxal = (log OR schizophrenia/per allele)/ βpyridoxal/per allele.
Results
Effect of rs4654748 on serum pyridoxal levels in nonpsychiatric Japanese control participants
We examined whether the C allele of rs4654748, which was identified in an Italian GWAS,3 was associated with serum pyridoxal levels in nonpsychiatric Japanese controls. A linear regression analysis showed a significant effect of rs4654748 on serum pyridoxal levels in the Japanese population (β = −0.13, standard error [SE] 0.05, p = 0.006).
Differences in serum pyridoxal levels between participants with and without schizophrenia
Median serum pyridoxal levels in 365 patients with schizophrenia and in 911 control participants were 4.4 ng/mL (range 2.0–91.1 ng/mL) and 8.9 (range 2.1–99.8 ng/mL), respectively. When we conducted a linear regression analysis to examine the effects of diagnosis, age, sex and the genotypes of rs4654748 on serum pyridoxal levels, we found significant effects of diagnosis, sex and rs4654748 genotype (diagnosis: p = 1.0 × 10−40; sex: p < 0.001; genotype: p < 0.001). A stratified analysis was performed to examine differences in serum pyridoxal levels between the 2 groups separately by sex and rs4654748 genotypes (a total of 6 strata), adjusting for age. A significant effect of diagnosis (lower in the schizophrenia group than in the control group) was observed in all strata (p < 0.006; Fig. 1). We did not detect pyridoxine and pyridoxamine (2 of the 3 forms of vitamin B6) in most participants. Among 1276 participants, 1248 exhibited a pyridoxine concentration below the lower limit of detection (3.0 ng/mL), and 1248 participants had a pyridoxamine concentration below the lower limit of detection (0.2 ng/mL).
Differences in serum pyridoxal levels between patients with schizophrenia and controls based on sex and rs4654748 genotypes.
A meta-analysis of case–control studies between serum pyridoxal levels and schizophrenia
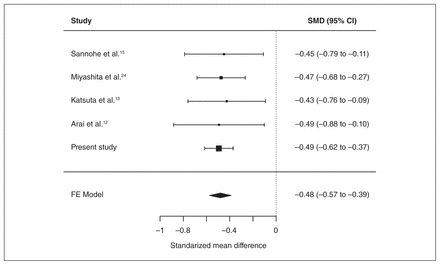
We performed a meta-analysis of previous association studies between serum pyridoxal levels and schizophrenia. The studies included in this meta-analysis are shown in Appendix 1, Table S2. We used 5 studies, including our data, for a total of 840 patients with schizophrenia and 1285 control participants. An inverse-variance fixed-effects model showed that serum pyridoxal levels were significantly lower in patients with schizophrenia than in controls (SMD −0.48, 95% confidence interval [CI] −0.57 to −0.39, p = 9.8 × 10−24); heterogeneity was not observed among studies (I2 = 0%, p > 0.99; Fig. 2). A funnel plot analysis indicated no evidence of a publication bias in the association studies (p = 0.76; Appendix 1, Fig. S2).
Meta-analysis of case–control studies between serum pyridoxal levels and schizophrenia. CI = confidence interval; FE = fixed-effects; SMD = standardized mean difference.
A meta-analysis of genetic association studies between rs4654748 and schizophrenia
We examined whether the C allele of rs4654748, which is associated with decreased pyridoxal levels, was a risk factor for schizophrenia in the Japanese population by conducting genetic association studies in 4 independent sample sets. The average genotype call rate was 99.5%, and the genotype concordance rate of 96 duplicate samples was 100%. The genotypic distributions of rs4654748 did not deviate significantly from Hardy–Weinberg equilibrium in the control group in all sample sets (all p > 0.05). No significant diagnostic differences were observed in the genotypic and allelic frequencies in these sample sets (all p > 0.05; Appendix 1, Table S3). Subsequently, we performed a meta-analysis of these 4 genetic association studies for a total of 4624 cases and 6010 controls. We observed no significant diagnostic differences in any genetic model (all p > 0.05; Appendix 1, Table S4), and no heterogeneity was noted among studies (all I2 = 0%, all p > 0.05).
Causality between serum pyridoxal levels and schizophrenia in a Mendelian randomization analysis
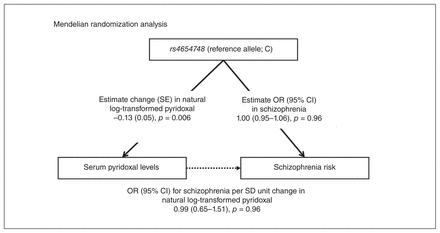
The βpyridoxal/per allele from the current association study between rs4654748 and serum pyridoxal levels in Japanese control participants (n = 859) was −0.13 (SE 0.05, p = 0.006). The estimated ORschizophrenia/per allele from the current meta-analysis of the Japanese genetic association studies between rs4654748 and schizophrenia (n = 10 634) was 1.00 (95% CI 0.95–1.06, p = 0.96). The estimated ORschizophrenia/pyridoxal calculated by combining the β pyridoxal/per allele and the ORschizophrenia/per allele were not significant, representing the OR of 0.99 (95% CI 0.65–1.51, p = 0.96; Fig. 3). When we calculated the estimated ORschizophrenia/pyridoxal by combining the pooled GWAS data sets, the βpyridoxal/per allele from a meta-analysis of pyridoxal GWAS (n = 1864),3 and the ORschizophrenia/per allele from a meta-analysis of schizophrenia GWAS (n = 150 064),23 we obtained the same result (ORschizophrenia/pyridoxal 0.99, 95% CI 0.98–1.01, p = 0.28).
Mendelian randomization analysis. CI = confidence interval; OR = odds ratio; SD = standard deviation; SE = standard error.
Association between pyridoxal and homocysteine
We examined the association between serum pyridoxal and plasma total homocysteine using our previous study’s homocysteine data.6 Although there was an inverse correlation between pyridoxal and homocysteine in the schizophrenia group (r = −0.11, p = 0.043), we did not find a significant inverse correlation between these variables in the control group (r = −0.06, p = 0.09).
Discussion
In this study, we first replicated an association between serum pyridoxal levels and rs4654748, which was identified in an Italian GWAS, in Japanese control participants. Next, we showed that serum pyridoxal levels were lower in the schizophrenia group than in the control group, with sex and rs4654748 genotype being other independently associated factors in the Japanese population. To our knowledge, this is the first case–control study separated by sex and by genotype associated with vitamin B6. Subsequently, we confirmed decreased serum pyridoxal levels in patients with schizophrenia by conducting a meta-analysis of association studies between serum pyridoxal levels and the disorder. This is also, to our knowledge, the first meta-analysis of cross-sectional studies of serum pyridoxal levels in schizophrenia. Our findings were consistent with the results of previous studies.12–15
Investigating causalities between serum pyridoxal levels and schizophrenia is important for understanding schizophrenia pathology and treatment. To our knowledge, this is the first MR study to examine the causal role of pyridoxal levels in schizophrenia. We evaluated the effect of long-term pyridoxal on schizophrenia by using a genetic variant associated with pyridoxal as an instrumental variable. We failed to obtain data supporting a causal relationship between decreased pyridoxal levels and schizophrenia risk in the Japanese population, as well as in the pooled GWAS data sets. Recently, we reported evidence of a causal relationship between elevated blood homocysteine levels and schizophrenia risk.6,7 When we examined the associations between plasma total homocysteine and serum pyridoxal levels in participants with schizophrenia and found a negative correlation between these 2 variables. These results suggest that decreased pyridoxal levels observed in patients with schizophrenia may be the result of the increased total homocysteine levels observed in indivdiuals with this disorder.
However, the absence of causality between serum pyridoxal levels and schizophrenia in our MR analysis must still be viewed with caution because several clinical studies have indicated that vitamin B6 may be involved in schizophrenia pathology and treatment. For example, Miyashita and colleagues24 identified a negative correlation between serum pyridoxal levels and symptom severity in patients with chronic schizophrenia. Katsuta and colleagues13 showed in a longitudinal study that decreased serum pyridoxal levels in patients with acute schizophrenia were normalized according to the clinical course, and the patients with decreased serum pyridoxal levels during the clinical course showed less improvement in symptoms. Furthermore, several previous studies reported the therapeutic effect of pyridoxine (1 of the 3 forms of vitamin B6) in patients with schizophrenia,25–30 although other studies, including a meta-analysis of randomized controlled trials of vitamin B6 alone,31 have not provided evidence of the benefits of pyridoxine in people with this disorder.32,33 These results suggest that pyridoxine may be effective for a particular subpopulation of patients with schizophrenia, but not for general schizophrenia populations.
Interestingly, a meta-analysis of randomized controlled trials showed that pyridoxal 5 phosphate (the active form of vitamin B6) may be effective in treating tardive dyskinesia in individuals with schizophrenia.34 Furthermore, the potential efficacy of vitamin B6 in the treatment of acute neuroleptic-induced akathisia and lithium-induced tremor has been reported.35–37 These results suggest that vitamin B6 may be involved in a broad spectrum of movement disorders.38
Limitations
Our study has several limitations that should be mentioned. First, most patients were treated with various antipsychotic drugs, and these medications may have influenced the results. However, we did not find any correlations between equivalent dose and serum pyridoxal levels in participants with schizophrenia, which is consistent with the findings of a previous study.13 Second, the blood samples used in the study were collected when patients visited the hospital; therefore, we cannot rule out that food consumption may have affected serum pyridoxal levels. Third, we did not take potential confounding factors, such as body mass index and metabolic parameters, into consideration in our analysis owing to lack of data. Additionally, limitations existed in our MR analysis, including sample size, a small proportion of variance in serum pyridoxal levels explained by rs4654748 (0.89%), and only 1 SNP that was used as an instrumental variable. Fifth, we performed the experiments on the participants with schizophrenia defined according to DSM-IV. Broader approaches, such as endophenotypes or dimensional syndrome models may be needed. Finally, we focused on pyridoxal concentrations in patients with schizophrenia in the present study. Vitamin B6 is a key component in one-carbon metabolism (Appendix 1, Fig. S3), and several components in one-carbon metabolism have been implicated in schizophrenia.4,5 An extensive systematic investigation of one-carbon metabolism will be needed to reveal the complex pathophysiology of schizophrenia.
Conclusion
We found decreased serum pyridoxal levels in patients with schizophrenia in this observational study. However, we failed to obtain data supporting a causal relationship between pyridoxal levels and schizophrenia using the MR approach. Further large studies, such as longitudinal epidemiological studies and randomized controlled trials, will be needed in the future.
Acknowledgements
The authors thank all of the volunteers who participated in this study as well as the physicians who helped collect clinical data and blood samples at the mental hospitals. The authors also thank Akemi Okada for her technical assistance and Tetsuo Ohnishi for his help for genotype analysis.
Footnotes
Funding: This work was supported in part by the Japan Agency for Medical Research and Development, AMED (T.O.), Grant-in-Aid for Scientific Research (C) (No.15K09809) (S.N.), and Grant-in-Aid for Young Scientists (B) (No.16K19768) (M.K.), and the grant from Research Group for Schizophrenia (S.N.), and the grant from SENSHIN Medical Research Foundation (M.K.).
Competing interests: None declared.
Contributors: S. Numata and T. Ohmori designed the study. Y. Tomioka, M. Kinoshita, H. Umehara, S. Watanabe, Y. Iwayama, T. Toyota, M. Ikeda, S. Shimodera and N. Iwata acquired the data, which Y. Tomioka, S. Numata, M. Kinoshita, M. Nakataki, H. Yamamori, A. Tajima, R. Hashimoto and T. Yoshikawa analyzed. Y. Tomioka and S. Numata wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received March 9, 2017.
- Revision received August 21, 2017.
- Revision received October 17, 2017.
- Accepted October 22, 2017.