Abstract
Background: Results of neuroimaging and postmortem studies suggest that people with schizophrenia may have lower levels of muscarinic M1 receptors (CHRM1) in the cortex, but not in the hippocampus or thalamus. Here, we use a novel immunohistochemical approach to better understand the likely cause of these low receptor levels.
Methods: We determined the distribution and number of CHRM1-positive (CHRM1+) neurons in the cortex, medial dorsal nucleus of the thalamus and regions of the hippocampus from controls (n = 12, 12 and 5, respectively) and people with schizophrenia (n = 24, 24 and 13, respectively).
Results: Compared with controls, levels of CHRM1+ neurons in people with schizophrenia were lower on pyramidal cells in layer III of Brodmann areas 9 (−44%) and 17 (−45%), and in layer V in Brodmann areas 9 (−45%) and 17 (−62%). We found no significant differences in the number of CHRM1+ neurons in the medial dorsal nucleus of the thalamus or in the hippocampus.
Limitations: Although diagnostic cohort sizes were typical for this type of study, they were relatively small. As well, people with schizophrenia were treated with antipsychotic drugs before death.
Conclusion: The loss of CHRM1+ pyramidal cells in the cortex of people with schizophrenia may underpin derangements in the cholinergic regulation of GABAergic activity in cortical layer III and in cortical/subcortical communication via pyramidal cells in layer V.
Introduction
Schizophrenia is a debilitating psychiatric disorder that affects approximately 1% of the population worldwide. Its peak age of onset is in early adulthood, and it is equally prevalent in urban and nonurban environments.1 Schizophrenia is diagnosed by the presence of specific constellations of symptoms, so the diagnosis encapsulates a syndrome of disorders with similar clinical presentations.2 While the cause of schizophrenia remains to be elucidated, it has been hypothesized that changes in central nervous system (CNS) cholinergic activity contribute to the pathophysiology of the disorder.3 Activation of the cholinergic system in the human CNS depends on 2 families of receptors4: muscarinic receptors, which are G-protein coupled, and nicotinic receptors, which are ligand-gated ion channels. There are 5 muscarinic receptors (CHRM1–5), and a neuroimaging study and some postmortem CNS studies have shown lower levels of muscarinic receptors in the CNS of people with schizophrenia.5,6 A number of postmortem studies have reported lower [3H]pirenzepine binding to cortical muscarinic receptors in people with schizophrenia7,8; [3H]pirenzepine is a radioligand that selectively binds to CHRM1 and CHRM4.7 Moreover, it has been shown that levels of CHRM1,9 but not CHRM2, CHRM310 or CHRM4,9 are lower in the cortex of people with schizophrenia. All these data suggest that people with schizophrenia have lower levels of cortical CHRM1, a receptor that is present in high numbers on glutamatergic pyramidal neurons but is mostly undetectable on GABAergic interneurons.11
Lower [3H]pirenzepine binding has been reported in the hippocampi of people with schizophrenia compared with controls,12,13 but mRNA data argue that it is levels of CHRM4, not CHRM1, that are lower in people with schizophrenia.13 This differentiation is important, because CHRM1 is postsynaptic in the hippocampus, whereas CHRM4 is pre- and postsynaptic. 14 Thus, cholinergic neurotransmission via projections from nuclei in the basal forebrain may be disrupted in the cortex and hippocampus in people with schizophrenia due to changes in different CHRMs. In contrast to findings in the cortex and hippocampus, levels of CHRM1 or CHRM4 in the thalamus do not differ between schizophrenia and controls. 15 This finding suggests that cholinergic transmission through the pedunculopontine-lateral dorsal tegmental projections of the brainstem into the thalamus may not be affected in people with schizophrenia.
Because the diagnosis of schizophrenia encapsulates a syndrome of disorders,2 progress toward understanding the pathophysiologies of its component disorders is hindered, at least in studies at the molecular and cellular levels. 16 With the concept of schizophrenia as a syndrome, we have identified a subset of people with schizophrenia (25%) who have a lower (−75%) density of [3H]pirenzepine binding sites in Brodmann area (BA) 9,17 and we have described this subgroup as having a muscarinic receptor deficit form of schizophrenia (MRDS). Importantly, under the binding conditions in our studies, [3H]pirenzepine binds to CHRM1 with a specificity greater than 80%,18,19 suggesting that levels of CHRM1 are markedly lower in the cortex of people with MRDS.
The availability of an anti-human CHRM1 antibody suitable for use with the postmortem human CNS20 allowed us to describe the distribution of CHRM1-positive (CHRM1+) neurons in the hippocampus.20 We have now measured the number of CHRM1+ neurons in postmortem CNS tissue from people with schizophrenia (both MRDS and non-MRDS) and controls. We studied BA9 (dorsolateral prefrontal cortex), because this was the region where we first identified the subgroup with MRDS.18 We also measured the distribution of CHRM+ neurons in BA17 (primary visual cortex), because the distribution of CHRM1 has been reported to differ from that in other cortical regions in primates.21 We also analyzed CHRM1+ neurons in the medial dorsal nucleus (MDN) of the thalamus (the location of the highest density of CHRM1 in the thalamus15) and regions of the hippocampus.
Methods
Collection and processing of human postmortem CNS
We obtained permission from the Ethics Committee of the Victorian Institute for Forensic Medicine to collect tissue postmortem. All tissue was collected after gaining written permission from next of kin. The approaches used to process tissue and collect relevant clinical data postmortem have been published previously.17,22,23
We obtained BA9, BA17, hippocampus and thalamus samples from the right hemisphere of the CNS, fixed in 10% formaldehyde for at least 2 weeks. We took BA9 from a region of the lateral surface of the frontal lobe and included the middle frontal gyrus superior to the inferior frontal sulcus. We took BA17 from the cortical region containing the band of Gennari and including the banks of the calcarine sulcus in the cuneus and lingua gyrus. We took hippocampus and thalamus from a coronal slice at the level of the lateral geniculate nucleus. After removal from formalin, tissue from each case was placed in phosphate-buffered saline before being serially sectioned at 50 mm on a vibratome and stored in phosphate-buffered saline containing 0.5% sodium azide at 4°C.
Immunohistochemistry
We performed immunohistochemistry using the Vectastain ABC peroxidase kit (Vector Laboratories, Inc.) as described previously20 (supplementary Methods, Appendix 1, available at jpn.ca/170202-a1). To avoid processing bias, in each region we selected the first section used from each case using a random number generator (www.random.org), and then sampled every 10th section after the first section. We took 5 sections from each case and incubated them with the primary antibody. We incubated 1 section without the primary antibody (negative control) and 1 with rabbit anti-glial fibrillary acidic protein antibody (1:4000, DAKO; Z0334; batch 20010594) as a positive immune-reaction control. To aid in the identification of cortical layers and hippocampal regions, we stained 1 section from each case using cresyl violet by placing the section in 0.1% cresyl violet acetate in deionized water for 20 minutes at room temperature, washing the section in deionized water, dehydrating the section through gradient alcohols, clearing the section in histolene and mounting the section in DPX.
We performed cell counting on an Eclipse 80i microscope (Coherent Scientific) with Stereo Investigator 10 (SciTec). After completing an automatic white balance, regions of interest were defined in each section using a 10 × objective. We used Stereo Investigator to randomize fields for sampling within regions of interest. Each section was stepped serially using the optical fractionator, with the section viewed under a 100 × objective lens with a numerical aperture of 1.4. The optical fractionator was implemented using upper and lower guard zones to enable a representative 3D counting frame for sampling cells, reducing z-axis bias from cells outside the plane of the section. In a similar manner, we used exclusion and inclusion boundaries to reduce bias from x- and y-axes. The dimensions of the dissectors were z = 12 μm, x = 105 μm and y = 52.5 μm. We used the nucleolus of neurons as a single point source for inclusion of the cell in all planes to further reduce bias in our estimates of cell densities.
We identified CHRM1+ neurons by the presence of clear immunopositivity, distinguishable from background, in the cell profile. We identified glia on the basis of a lack of stained cytoplasm, a more rounded appearance and the presence of heterochromatin. We identified neurons by the presence of a distinct nucleolus.24
Statistical analysis
We identified group differences using the Student t test or an analysis of variance with a post hoc Dunnett test comparison to controls. We used Pearson product-moment correlations to identify relationships between experimental data and case demographics. Pharmacological or tissue collection data with strong correlations (r2 > 0.49, p < 0.0525) were an indicator for an analysis of covariance, with the appropriate parameters as covariates. We compared the frequency of variables in non-continuous data sets using the Fisher exact test and analyzed experimental data to determine whether they were associated with variations in experimental measures.
Results
Case demographics, CNS collection and pharmacology
Tissue from the cortex and thalamus was available for all cases (Table 1 and Appendix 1, Table S1). There were no significant variations in mean age, postmortem interval, CNS pH or sex ratio by diagnosis (Table 1). When we divided the schizophrenia group into MRDS and non-MRDS subgroups, we found no significant variations in mean age, postmortem interval, CNS pH or sex ratio. Mean duration of illness, final recorded antipsychotic drug dose, frequency of cases exposed to muscarinic receptor antagonists and rates of suicide did not differ between people with MRDS and non-MRDS. However, in people with schizophrenia at the level of the syndrome, and in people with MRDS, levels of [3H]pirenzepine binding were lower than in controls (Fig. 1).
(A) A photomicrograph showing anti-muscarinic M1 receptor antibody immunolabelled cells in Brodmann area 9 (arrows highlighting CHRM+ neurons). (B) An adjacent tissue section that was treated identically but not exposed to the anti-muscarinic M1 receptor antibody. (C) Levels (mean ± SEM) of [3H]pirenzepine binding to Brodmann area 9 for the cases included in this study that were part of a larger published cohort.17 (D–G) Levels (mean ± SEM) of CHRM1+ neurons, total neurons and total glia in (D) layer III and (E) layer V in Brodmann area 9 and (F) layer III and (G) layer V in Brodmann area 17 from people with schizophrenia, a subgroup of people with MRDS and people with schizophrenia who did not have that deficit (non-MRDS). a = p < 0.05, b = p < 0.01, c = p < 0.001, d = p < 0.0001. CHRM+ = muscarinic M1 receptor–positive; MRDS = muscarinic receptor deficit form of schizophrenia; SEM = standard error of the mean.
Demographic, antipsychotic treatment, anticholinergic drug treatment and central nervous system collection characteristics
Hippocampal tissue was available from only 13 people with schizophrenia and 5 controls (Table 1). People with schizophrenia were younger than the controls (Table 1), but mean postmortem interval, CNS pH and sex ratio did not vary by diagnosis. When the schizophrenia group was divided into MRDS (n = 6) and non-MRDS (n = 7) subgroups, mean age, postmortem interval, CNS pH and sex ratio did not vary by group. Mean duration of illness, final recorded antipsychotic drug dose, frequency of cases exposed to muscarinic receptor antagonists and rates of suicide did not differ between people with MRDS and non-MRDS.
Immunohistochemistry
As in the hippocampus,20 immunolabelling was present on specific neurons in the cortex (Fig. 1A) and the MDN (Fig. 2A). We found no immunolabelling of neurons when the anti-CHRM1 antibody was omitted (Fig. 1B). As in the hippocampus, 20 immunolabelling was diffuse in the cell bodies in both the cortex and MDN, consistent with the presence of CHRM1 in the cytosol as well as on neuronal membranes.
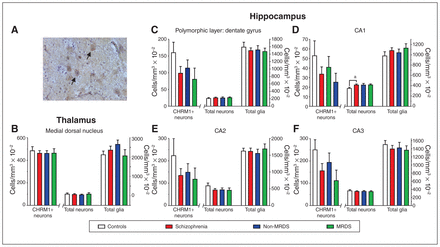
(A) A photomicrograph showing antimuscarinic M1 receptor antibody immunolabelled cells in the medial dorsal nucleus (arrows highlighting examples of CHRM+ neurons). (B–F) Levels (mean ± SEM) of CHRM1+ neurons, total neurons and total glia in (B) the medial dorsal nucleus, (C) the polymorphic layer of the dentate gyrus, (D) CA1, (E) CA2 and (F) CA3 from people with schizophrenia, a subgroup of people with MRDS and people with schizophrenia who did not have that deficit (non-MRDS). a = p < 0.05. CA = cornu ammonis; CHRM1+ = muscarinic M1 receptor–positive; MRDS = muscarinic receptor deficit form of schizophrenia; SEM = standard error of the mean.
Compared with controls, the number of CHRM1+ neurons was lower in layers III and V of BA9 in people with schizophrenia (−44% and −45%, respectively), MRDS (−54% and −57%, respectively) and non-MRDS (−33% and −33%, respectively; Fig. 1D and 1E; see Appendix 1, Table S2, for details of full statistical analyses). In BA17, we found lower numbers of CHRM1+ neurons in layers III and V from people with schizophrenia (−45% and −62%, respectively; Fig. 1F and 1G; Appendix 1, Table S2). In layer III, the number of CHRM1+ neurons was lower in people with MRDS (−62%), but not in people with non-MRDS (−28%), whereas in layer V the number of CHRM1+ neurons was lower in people with MRDS (−79%) and non-MRDS (−45%; Appendix 1, Table S2). The total number of neurons or glia in BA9 and BA17 did not differ between controls and people with schizophrenia, MRDS or non-MRDS (Fig. 1D to G; Appendix 1, Table S2). We also completed a post hoc power analysis of data on CHRM1+ cells in layer III in BA17 from people with non-MRDS and controls, which showed that if there had been 53 cases in each cohort, the difference in numbers of CHRM1+ neurons could have reached a significance of p < 0.05. This raised the possibility that the absence of change in CHRM1+ neurons in BA17 from people with non-MRDS could have resulted from a lack of power in this study.
We focused our studies on the MDN, where there was a sufficient number of CHRM1+ neurons to give meaningful counts. We found no differences in numbers of CHRM1+ neurons, total neurons or total glia between patient groups and controls (Fig. 2B; Appendix 1, Table S2).
In the hippocampus, the density of cells in the molecular layer of the dentate gyrus made identifying and counting CHRM1+ neurons impossible. In the cornu ammonis (CA) 1, CA2, CA3 and the polymorphic layer of the dentate gyrus, we found no differences in number of CHRM1+ neurons (Fig. 2 C to F; Appendix 1, Table S2). The total number of neurons — but not total glia — was higher (+19%) in CA1 from people with schizophrenia, but not in MRDS or non-MRDS. In contrast, the number of total neurons and total glia did not differ between groups in CA2, CA3 or the polymorphic layer of the dentate gyrus.
Potential confounders
In the hippocampi of men, the total number of neurons was lower in the polymorphic layer of the dentate gyrus (−22%), CA2 (−18%) and CA1 (−13%), but the total number of glia was higher in the polymorphic layer of the dentate gyrus (+ 13%; Table 2). However, given the low number of cases from which hippocampus was available, this finding must be viewed as preliminary. This is particularly the case because power analyses suggested that larger cohorts could have resulted in findings of lower CHRM1+ neurons in people with MRDS and non-MRDS in all regions but CA1 (Appendix 1, Table S4).
The effect of sex and suicide (schizophrenia only) on levels of muscarinic receptor–positive neurons, total neurons and total glia in Brodmann areas 9 and 17, medial dorsal nucleus and regions of the hippocampus*
In the MDN, the numbers of CHRM1+ neurons and total neurons were lower (−28%) in people with schizophrenia who died by suicide (n = 7) than in those who died of other causes (n = 17; Table 2).
We observed some associations between experimental data and demographic, CNS collection or pharmacological data where the best fit linear regression deviated significantly from zero, but none of these was of sufficient strength to warrant a secondary analysis (Appendix 1, Table S3).
In addition, when comparing results from people with schizophrenia who had or had not been treated with anticholinergic drugs, we found no differences in the levels of CHRM1+ neurons, total neurons or total glia in layer III or layer V in BA9 or BA17 (Table 3).
Levels of CHRM1+ neurons, total neurons and total glia in layers III and V in Brodmann areas 9 and 17 from people with schizophrenia who had or had not been prescribed anticholinergic medication in life
Discussion
We have reported lower levels of CHRM1+ neurons in layer III and layer V in the BA9 and BA17 of people with schizophrenia compared with controls, but no significant differences in the number of CHRM1+ neurons in the MDN or in CA1, CA2, CA3 or the polymorphic layer of the dentate gyrus in the hippocampus. Given that CHRM1 is a potent regulator of cholinergic activity in the mammalian cortex,26 our data suggest widespread dysregulation of cortical cholinergic functioning in people with schizophrenia. We know that CHRM1s are found predominantly on pyramidal cells in layers III and V of the cortex, where they are involved in cell-autonomous molecular alterations in layer III pyramidal cells, a process that is thought to be affected in people with schizophrenia and a contributor to cortical GABAergic dysfunction.27 This could mean that CHRM1 on layer III pyramidal cells causes changes in the glutamatergic control of GABAergic neurons in people with schizophrenia.28
Pyramidal cells in layer V of the cortex receive axonal input from the cortex and have projections to regions such as the midbrain, the basal ganglia of the forebrain and the spinal cord. Hence, our data also support the notion that a loss of CHRM1 on layer V pyramidal cells deranges the cortical/subcortical circuitry that is postulated to occur in people with schizophrenia.29
This study uniquely examines the number of CHRM1+ neurons in people with MRDS who have significantly lower levels of [3H]pirenzepine binding to CHRM1 + CHRM47 compared with controls and non-MRDS. We have shown that people with MRDS have lower levels of CHRM1+ neurons in layers III and V of BA9 and BA17, but not in the MDN or the hippocampus. In people with non-MRDS, levels of CHRM1+ neurons were lower in layers III and V in BA9 and layer V in BA17, but not in layer III of BA17, MDN or the hippocampus. Notably, we found no difference in the total number of neurons in BA9 or BA17 from people with MRDS and non-MRDS. In MRDS, these data were consistent with the concept of fewer neurons expressing CHRM1 rather than a loss of CHRM1+ neurons through degenerative processes. In non-MRDS, it would seem most likely that while fewer neurons expressed CHRM1, the CHRM1+ neurons made enough receptors that CHRM1 receptor density, as measured by [3H]pirenzepine binding, did not differ from controls. Predicting the functional outcomes of these changes is more difficult, but it is possible that in MRDS there is a deficit in cholinergic signal amplitude because of a lack of CHRM1, and a loss of cholinergic modulation of pyramidal cell activity because more of these cells appear to lack CHRM1. In contrast, if fewer CHRM1+ neurons are expressing higher levels of CHRM1s in the cortices of people with non-MRDS, this could result in a higher cholinergic signal amplitude on the pyramidal cells that express CHRM1 and an absence of cholinergic signalling on pyramidal cells that do not. This could result in a different pattern of CHRM1+ signalling in the cortex of people with non-MRDS and may have subtle effects on cholinergic activity that could still affect cortical function.
We found no changes in total numbers of neurons or glia in layers III or V in BA9 and BA17 from people with schizophrenia, MRDS or non-MRDS. These data showed some similarities to a study that reported lower levels of glutamic acid decarboxylase–expressing neurons with no change in total neuron count in the cortex of people with schizophrenia. 30 Another study has reported lower neuronal density in layer VI in the prefrontal cortex, layer V of the cingulate cortex and layer III of the motor cortex,31 suggesting that neuronal loss may be cortical layer × region–specific. Our findings do not contradict this hypothesis, but argue that there is no change in neuronal density in layers III and V in BA9 and BA17, consistent with a study reporting no loss of neuronal density on layers III and V in BA9 from people with schizophrenia. 32 In contrast, our findings do not agree with a study that reported an increase in neuronal density in BA9 and BA17 from people with schizophrenia.33
We found no significant difference in the number of CHRM1+ neurons in the MDN of people with MRDS or non-MRDS, adding to the data showing no change in levels of [3H]pirenzepine binding in the thalamus of people with schizophrenia.15 This suggests that the thalamus is spared the loss of CHRM1s and CHRM1+ neurons. As in a previous study,34 we failed to show any changes in neuronal or glial density in the MDN of people with schizophrenia.
This study showed no change in the number of CHRM1+ neurons in CA1, CA2, CA3 and the polymorphic layer of the dentate gyrus from people with schizophrenia, MRDS or non-MRDS. However, this finding must be regarded as preliminary because of the small number of cases for which we had suitable hippocampal tissue. However, at present our data on CHRM1+ neurons in people with MRDS and non-MRDS would support the argument that lower levels of CHRM4 are causing decreased [3H]pirenzepine binding in the hippocampus of people with schizophrenia.13 We did find a small (19%) but significant increase in total neurons in CA3 of people with schizophrenia that was not apparent when we divided the group into MRDS and non-MRDS subgroups. These data partly replicate a previous study that found increased neuronal density in CA3 and CA1 of people with schizophrenia.35
We also found lower numbers of CHRM1+ neurons and total neurons in the MDN of people who died by suicide compared with those who died of other causes. Notably, we did not observe changes in [3H]pirenzepine binding in the MDN or other regions of the thalamus from people who died by suicide compared with death from other causes.15 Hence, although the thalamus is a CNS region of interest with respect to suicidal ideation,36 our data were unclear about whether changes in CHRM1-mediated activity in the thalamus may contribute to a propensity for suicidal ideation.
Limitations
Our study had some limitations, but they did not include the use of an antibody that lacks specificity for CHRM1, which is a concern for some anti-muscarinic M1 receptor antibodies.20 Our findings in the hippocampus are preliminary because of relatively small cohort sizes. In addition, like all studies that include living or dead people with schizophrenia who have been treated, it is possible that changes may have been due to antipsychotic drug treatment. While we7,12 and others37,38 have shown that treating rats with antipsychotic drugs does not lower levels of cortical or hippocampal [3H]pirenzepine binding, we are aware of no studies that examine the density of CHRM1+ neurons after such treatment. However, treating rats with muscarinic receptor antagonists does not affect cortical [3H]pirenzepine binding.7 In addition, there was no difference in levels of CHRM1+ neurons, total neurons or total glia in people with schizophrenia who had received anticholinergic drugs compared to those who had not been treated with such drugs, supporting the notion that treatment with that class of drugs does not decrease levels of CHRMs.
Conclusion
Our human data and studies of mammalian cortex show that CHRM1s are located primarily on pyramidal neurons,11 which are strongly innervated by cholinergic neurons.39 These pyramidal cells are important in regulating mental representations that subserve higher cognitive processing,40 and cortical CHRM1s are critical in maintaining cognitive function in humans.41 Thus, while activating cortical CHRM1 with orthosteric or allosteric modulators could provide therapeutic benefits in people with schizophrenia,42,43 the loss of cortical CHRM1s and CHRM1+ neurons in BA9 and BA17, and possibly other areas of the cortex, could have serious implications for the ability of such drugs to help normalize cholinergic neurotransmission in people with schizophrenia, particularly MRDS. Adding to these concerns are our data showing that cortical tissue from people with MRDS (compared with control and non-MRDS) is less responsive to CHRM1 orthosteric agonists44 and positive allosteric modulators. 45 Therefore, a neuroimaging test to identify people with low levels of muscarinic receptors before treatment with muscarinic-receptor-activating drugs may help to show whether low levels of CHRM are associated with resistance to treatment with CHRM1-targeting drugs.
Our data showing lower levels of cortical CHRM1+ neurons in people with schizophrenia adds weight to the hypothesis that abnormalities in cortical pyramidal cell activity in cortical layers III and V contributes to the pathophysiology of the disorder. Because CHRM1s41 and cortical pyramidal cells21 are important in maintaining cognitive function, we hypothesize that the loss of cortical CHRM1+ neurons likely contributes to the cognitive deficits experienced by many people with schizophrenia.46 In addition, CHRM1s in BA17 are important in modulating visual attributes such as form, position and motion40; our findings on the loss of CHRM1+ neurons in that cortical region in people with schizophrenia could suggest abnormalities in assigning visual attributes and may be a contributing factor to the visual-related symptoms associated with the disorder.
Acknowledgements
The authors thank the staff of the Victorian Brain Bank Network and the Advanced Microscopy Facility, the Florey, for their support. Tissues were received from the Victorian Brain Bank Network, supported by the Florey, the Alfred Hospital and the Victorian Institute for Forensic Medicine. This work was supported by the National Medical and Health Research Council (grants APP1048544, APP1045619, APP1002240 and APP1037234), the Australian Research Council (grants DP110100086 and FT100100689), the Andrew and Claire Henna Ride for Ben, One in Five and the Operation Infrastructure Support Grant from the Victorian State Government.
Footnotes
Competing interests: None declared.
Contributors: E. Scarr, I. Everall, T. Aumann, G. Chana and B. Dean designed the study. E. Scarr, S. Hopper, V. Vos and M. Seo acquired and analyzed the data, which B. Dean also analyzed. B. Dean wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received October 19, 2017.
- Revision received November 28, 2017.
- Revision received December 19, 2017.
- Revision received December 20, 2017.
- Accepted January 4, 2018.