Abstract
Background: Childhood abuse is associated with structural brain abnormalities. Few studies have investigated white matter tract abnormalities in medication-naive, drug-free individuals who experienced childhood abuse. We examined the association between childhood abuse and abnormalities in white matter tracts in that population, controlling for psychiatric comorbidities.
Methods: We collected diffusion tensor imaging data for age- and sex-matched youth with childhood abuse, psychiatric controls (matched for psychiatric diagnoses) and healthy controls. Tract-specific analysis was conducted using tractography. Tract-based spatial statistics (TBSS) was used to assess group differences in fractional anisotropy (FA) at the whole-brain level.
Results: We included 20 youth who experienced childhood abuse, 18 psychiatric controls and 25 healthy controls in our analysis. Tractography analysis showed abuse-specific reduced tract volume in the inferior longitudinal fasciculus (ILF) and inferior frontal-occipital fasciculus (IFoF) in the abuse group relative to both healthy and psychiatric controls. Furthermore, abnormalities in the left IFoF were associated with greater abuse severity. The TBSS analysis showed significantly reduced FA in a left-hemispheric cluster comprising the ILF, IFoF and corpus callosum splenium in the abuse group relative to healthy and psychiatric controls.
Limitations: It is unclear to what extent pubertal development, malnutrition and prenatal drug exposure may have influenced the findings.
Conclusion: Childhood abuse is associated with altered structure of neural pathways connecting the frontal, temporal and occipital cortices that are known to mediate affect and cognitive control. The abuse-specific deficits in the ILF and IFoF suggest that fibre tracts presumably involved in conveying and processing the adverse abusive experience are specifically compromised in this population.
Introduction
Brain development is a complex process that is regulated by genes and sculpted by environmental experiences. Although experiential influences affect brain structure and function throughout the lifespan, early childhood experience is particularly crucial, as early stress and exposure to traumatic events have been shown to adversely affect the nature and trajectory of normal brain development.1
Childhood maltreatment, which includes physical, sexual and emotional abuse and neglect, is common in the United Kingdom, with pediatric prevalence of 7%–10%.2 It has been associated with a host of adverse consequences, such as low IQ, abnormal error processing,3 and impaired attention, inhibition, emotion and reward processing.4,5 Large-scale epidemiological studies found that childhood maltreatment was significantly associated with onset of various psychiatric disorders, such as depression and posttraumatic stress disorder (PTSD).6 The psychopathological outcomes associated with childhood maltreatment may be mediated by the disruption of neural underpinnings.7
Structural MRI studies show that childhood maltreatment is associated with grey-matter volume abnormalities in several relatively late-developing brain regions, particularly the orbitofrontal cortex (OFC)8–10 and temporal lobes11,12 as well as the visual cortex.8,13,14 Our meta-analysis of voxel-based morphometry studies showed that childhood maltreatment is associated with grey-matter volume reduction in OFC–limbic–temporal regions and inferior frontal cortices that mediate top–down affect and cognitive control, respectively, and in the left motor and somatosensory cortices that mediate sensory functions.10
Compared with the extensive research on grey-matter volume abnormalities in childhood maltreatment, fewer studies have examined white-matter tracts in this population. Brain regions do not function independently; they are interconnected through a complex system of short- and long-range white-matter tracts.15 White matter connectivity regulates the speed and timing of activation across neural networks, which are essential for optimal performance of higher-order tasks that rely on integrated information processing.16
Diffusion tensor imaging (DTI) measures the restricted diffusion of water molecules and provides a more detailed assessment of fibre tracts than conventional MRI and has emerged as a powerful technique for examining structural connectivity.17 Fractional anisotropy (FA), a DTI-derived metric, describes the directionality of water diffusion and may reflect aspects of membrane integrity and myelin thickness, where decreased FA is usually associated with white-matter disruption.18 Tractography facilitates the reconstruction of 3-dimensional trajectories of specific white-matter tracts and probes their microstructure, which allows a more detailed analysis of specific subpopulations of fibres and indirect volumetric indices (e.g., number of streamlines and tract volume). 19 These volumetric indices can be indicative of the speed of communication between different brain regions. Tract-based spatial statistics (TBSS), on the other hand, is a fully automated approach that permits a whole-brain analysis of white matter in a voxel-wise manner, which allows the identification of white-matter differences in specific regions beyond a priori–defined tracts.20 Therefore, we used these complementary methods to examine atypical white-matter tracts in youth exposed to childhood abuse.
Stress can affect white-matter tract development, as corticosteroids can suppress the final mitosis of glial cells necessary for myelination.21 Moreover, given the protracted postnatal development timeline of white matter,22 it may be particularly vulnerable to the neurotoxic impact of childhood trauma, especially during certain sensitive periods. Several DTI studies reported that childhood maltreatment was associated with reduced FA in various large white-matter tracts, particularly the inferior fronto–occipital fasciculus (IFoF), which is a direct pathway connecting the occipital, posterior temporal and the OFC areas;23–25 the inferior longitudinal fasciculus (ILF) connecting the occipital with the anterior temporal cortex,23,26,27 which is considered to be an indirect pathway connecting similar brain areas as the IFoF and anteriorly joins the uncinate fasciculus to relay information to the OFC; the superior longitudinal fasciculus (SLF) connecting the Broca and Wernicke areas;24,25,27 the corpus callosum (splenium) connecting the (posterior) left and right cerebral hemispheres;24,25 and the uncinate fasciculus connecting the anterior temporal lobe with the medial and lateral OFC.28
Given that childhood maltreatment is associated with the development of psychiatric complications,29 it is crucial to control for these in order to disentangle the effects of maltreatment from psychiatric comorbidities.10 So far, only 3 DTI studies included a psychiatric group without childhood maltreatment; 25,30,31 however, those studies used adult samples and focused only on depression, which limits the generalizability of their findings to other psychiatric comorbidities. Furthermore, a number of DTI studies have not measured and/or controlled for drug abuse23,28 and medication use,23,26–28,30 which are known to affect brain structure.32
The aim of the present study was to examine the association between childhood abuse and white-matter tract abnormalities by conducting tract-specific and whole-brain analyses in medication-naive, drug-free youth with documented childhood physical abuse compared with healthy controls. To assess the specificity of the association with abuse, we included a third group of psychiatric controls that was matched with the abuse group on psychiatric comorbidities. Sexual abuse was excluded because it has different effects on brain structure33 and different behavioural and psychiatric consequences.34 It has also been argued that childhood sexual abuse is associated with experiences unique to sexual victimization relative to other abuse experiences; for example, traumatic sexualization, betrayal, stigmatization as well as feelings of guilt and shame may affect victims of sexual abuse differently than victims of other abuse experiences.35 For these reasons, and in order to obtain a more homogeneous group, we included youth exposed only to childhood physical abuse. Nevertheless, it is unrealistic to separate physical abuse from typically co-occurring emotional abuse and neglect because psychological maltreatment would be present in almost all cases of physical maltreatment.36 Hence, it is unlikely that the abused victim would experience severe physical abuse without experiencing at least moderate levels of emotional abuse and neglect concurrently; however, physical abuse does not always co-occur with sexual abuse.
Given that childhood maltreatment is associated with grey-matter volume deficits in OFC–limbic–temporal and occipital visual regions,8,10,13,14 along with abnormalities in the white-matter tracts connecting these regions,23–27 we hypothesized that the abuse group would have white-matter tract abnormalities, particularly of the IFoF and ILF, relative to both the healthy and psychiatric control groups. We also investigated atypical FA in regions beyond our a priori–defined tracts with a whole-brain TBSS analysis.
Methods
Participants
Youth with childhood abuse, psychiatric controls and healthy controls who were right-handed, medication-naive, drug-free and matched for age and sex were assessed by a child psychiatrist (K.M.) using the Development and Well-Being Assessment (DAWBA),37 designed to generate ICD-10 and DSM-IV psychiatric diagnoses. The Strengths and Difficulties Questionnaires (SDQ)38 and Beck Depression Inventory (BDI)39 were also used to provide symptom scores on psychopathology. We assessed IQ using the Wechsler Abbreviated Scale of Intelligence (WASI).40 The Childhood Trauma Questionnaire (CTQ)41 was used to measure the severity of childhood physical, sexual and emotional abuse as well as physical and emotional neglect. Socioeconomic status (SES) was measured by 2 nonsensitive items (on housing tenure and room occupancy) from the Family Affluence Scale (FAS).42
The 23 youth who experienced physical abuse before the age of 12 years were first recruited through social services and psychiatric clinics. They or their guardians were first asked to provide signed permission to contact social services for written confirmation of official records of physical abuse. The Childhood Experience of Care and Abuse (CECA) interview43 was used to corroborate the CTQ and provide additional information including the age at onset and duration of abuse. Participants scored 13 or higher (i.e., the cut-off for severe/extreme physical abuse)41 on the CTQ physical abuse subscale, and information from the CECA interview and the CTQ were consistent with the official records. Common psychiatric comorbidities included PTSD, depression, anxiety and conduct disorder.
The 20 psychiatric patients who were matched with the abuse group on psychiatric comorbidities but who had no history of childhood maltreatment (scoring below the cut-offs for the respective CTQ subscales)41 were recruited through psychiatric clinics and social services. Patients with PTSD experienced non-abuse-related trauma (e.g., witnessed a murder, experienced a car accident or the death of a loved one).
Participants in the childhood abuse and psychiatric control groups who were recruited from social services did not have any psychiatric diagnoses beforehand, and their family physicians were subsequently notified by the child psychiatrist (K.M.). Those who were recruited from clinics were new clinical cases and had not yet started any treatment, and the diagnoses made using the DAWBA were consistent with the patients’ diagnoses in the clinics. None of the participants was receiving any treatment at the time of recruitment and scanning.
The 27 healthy controls with no history of psychiatric illness and childhood maltreatment (scoring below the same cut-offs for the respective CTQ subscales) were recruited through advertisements in the same geographic areas of South London to ensure similar socioeconomic background.
Exclusion criteria for all participants were childhood sexual abuse, drug abuse, learning disability, neurologic abnormalities, epilepsy, IQ below 70 and MRI contraindications. Urine screening for recent drug use was conducted with 10-panel urine drug test integrated cups (T-Cup; Testfield). Participants were also asked about drug use in the previous 4 weeks; most did not use any drugs in the last 4 weeks before the scan and there were no significant group differences (Appendix 1, Table S1, available at jpn.ca/170241-a1). All participants, or their guardians if they were younger than 18 years, provided written informed consent to participate in the study. The study was approved by the local NHS Research Ethics Committee.
Image acquisition and processing
The DTI acquisition procedures are described in Appendix 1. Diffusion data were preprocessed using ExploreDTI (www.exploredti.org).
We assessed group differences in head motion, as this may affect quantitative diffusion measurements. We quantified head motion as the mean volume × volume translation and rotation. This was calculated as the average across the translation or rotation component of the affine registration performed between each volume and the first volume, and t tests were then performed between the 2 groups for each of the 2 motion measures. As there were no significant group differences in mean translation (F2,60 = 0.8, p = 0.45) or rotation (F2,60 = 2.2, p = 0.1), we did not use motion as a nuisance regressor in our results.
Outlier profiles of each diffusion scan were generated using ExploreDTI during the quality check stage of preprocessing, with no difference between groups observed (F2,60 = 1.20, p > 0.05). All scans were then corrected for head motion using ExploreDTI.
Tractography
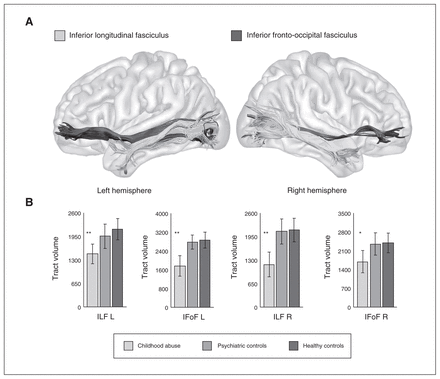
We performed virtual dissections of the left and right ILF and IFoF according to previous studies19 (Fig. 1). Regions of interest (ROIs) were delineated in the FA maps of each participant in native space using previously described anatomic guidelines to constrain the whole-brain tractogram.19 Two ROI approaches were used for each tract to show the full extent of white-matter streamlines running through each ROI. Specifically, the ILF was dissected to show streamlines running between the occipital lobe (1 ROI in the coronal plane within the white matter of the occipital lobe) and the temporal pole (1 ROI in the coronal plane within the white matter of the anterior temporal lobe). The IFoF was dissected using the same occipital lobe ROI as used for the ILF and a second ROI delineated in the coronal plane within the external capsule.
(A) Tractography reconstructions of the inferior longitudinal fasciculus (ILF) and inferior fronto-occipital fasciculus (IFoF) tracts. (B) Differences in the tract volume of the ILF and IFoF between the childhood abuse group, psychiatric controls and healthy controls. Statistically significant differences between the childhood abuse group and psychiatric and healthy control groups within each tract are indicated with asterisks (*p < 0.05; **p < 0.01).
Group differences were examined for each measurement (i.e., streamline count, tract volume, FA, mean diffusivity and radial diffusivity) using analysis of variance (ANOVA) with SPSS software (SPSS, Inc.), controlling for IQ, age and sex. Comparisons for specific tracts were considered to be statistically significant if they survived Bonferroni correction for multiple comparisons (p < 0.0125, 2 tracts for each hemisphere).
Tract-based spatial statistics
Each participant’s FA map was transformed into standard stereotactic space (using the FMRIB58 template), and a mean FA map for the whole sample was used to create the average core “skeleton.” Skeleton images of each participant’s FA map were then produced and projected onto the mean skeleton using a general linear model to identify voxels where FA value differed significantly among these skeletons.20 The design matrix used IQ, age and sex as covariates. Five thousand permutations were applied. The statistical threshold was set at p < 0.05, fully corrected for multiple comparison using threshold-free cluster enhancement (TFCE) across all white-matter tracts in the whole-brain analysis.
Exploratory correlational analysis
Finally, Pearson correlations were used to explore possible associations between tract-specific measurements and SDQ within each group and with abuse measures (severity, age at onset and duration of abuse) within the abuse group.
Results
Participants
We included 63 youth in our analyses. Of the 23 youth recruited to the abuse group, 3 were excluded owing to MRI motion artifacts, leaving a final sample of 20 participants in that group. Of the 20 recruited psychiatric controls, 2 were excluded owing to motion artifacts, leaving a final sample of 18 patients in that group. Of the 27 healthy controls recruited, 2 were excluded due to motion artifacts, leaving a final sample of 25 participants in that group. The demographic and clinical characteristics of participants are shown in Table 1.
Demographic characteristics of 20 youth exposed to childhood abuse, 18 psychiatric controls and 25 healthy controls
The groups did not differ significantly in age, sex, race or SES, but they differed in IQ, which was expected as this is typical for these populations44 (Table 1). Participants in the childhood abuse group did not mention any head trauma injuries or loss of consciousness from the abuse in the CECA interview. All MRIs were also reviewed by a radiologist, and no traumatic brain injury or incidental findings were discovered. Hence, mild traumatic brain injury is unlikely to affect the findings. Although we selected participants with severe childhood physical abuse, they also experienced marked/severe emotional abuse and neglect (Table 1), which typically co-occur with physical abuse; hence, they seem to adequately represent the childhood abuse population.36
The healthy controls scored significantly lower than the abuse group on the BDI (p < 0.01) and all SDQ difficulties subscales (p < 0.01), and they scored lower than psychiatric controls on the BDI (p < 0.001) and all SDQ difficulties subscales (p < 0.05) except for SDQ conduct problems. The abuse group scored significantly higher than psychiatric controls, who did not differ from healthy controls, on the SDQ conduct problems subscale (p < 0.01; Table 1).
Tractography analysis
The abuse group had significantly lower tract volume of the left ILF, right ILF and left IFoF than both healthy (p < 0.01) and psychiatric controls (p < 0.01) (Table 2, Fig. 1); lower streamline count of the right ILF and left IFoF than both healthy (p < 0.01) and psychiatric controls (p < 0.01); and lower FA of the left IFoF than healthy controls (p = 0.01) (Table 2). There were no significant differences between the healthy and psychiatric controls.
Measurements of the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus tracts
Tract-based spatial statistics analysis
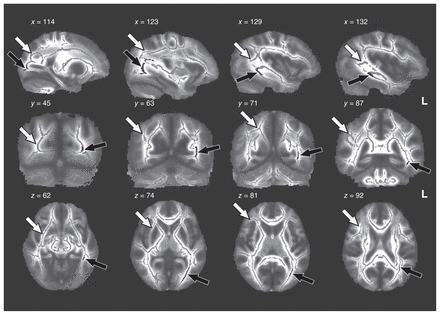
The abuse group, relative to healthy controls, had significantly reduced FA in a left-hemispheric posterior region comprising the ILF, IFoF, splenium of the corpus callosum and the SLF (p = 0.02, TFCE-corrected) (Table 3, Fig. 2). Mean FA values of this region were extracted for comparison between the abuse and psychiatric groups using ANOVA with SPSS24, controlling for IQ, age and sex. The abuse group had significantly reduced FA relative to psychiatric controls (F1,36 = 16.4, p < 0.001), which suggests that compromised microstructure of this region may be abuse-specific. The psychiatric controls had marginally lower FA than healthy controls in this region (F1,41 = 3.89, p = 0.06). There were no significant regions with increased FA for the abuse versus healthy and psychiatric groups.
Whole-brain tract-based spatial statistics analysis of differences in fractional anisotropy FA values between the childhood abuse group and healthy controls (p < 0.05, TFCE-corrected). Sagittal, coronal and transversal axial sections of the white matter skeleton (white arrows) superimposed on the mean FA brain template. Black arrows indicate regions with significantly reduced FA values in the abuse group compared with healthy controls. The x, y, z coordinates are in standard Montreal Neurological Institute space. Images are in radiological convention (The right side of the image corresponds with the left hemisphere of the brain and vice versa). FA = fractional anisotropy; TFCE = threshold-free cluster enhancement.
Cluster of reduced white matter fractional anisotropy in the childhood abuse group compared with healthy controls (p < 0.05, TFCE-corrected)
Exploratory correlational analysis
Reduced FA of the left IFoF was significantly associated with higher CTQ physical neglect (r = −0.52, p < 0.05), emotional neglect (r = −0.48, p < 0.05) and CTQ total score (r = −0.50, p < 0.05) within the abuse group (Appendix 1, Fig. S1). For the healthy controls, FA of the lower left IFoF was significantly associated with higher SDQ emotion (r = −0.61, p < 0.05) and peer (r = −0.46, p < 0.05) problems and SDQ total score (r = −0.51, p < 0.05). Lower left ILF tract volume was significantly associated with higher SDQ peer (r = −0.67, p < 0.05) and hyperactivity (r = −0.69, p < 0.05) problems and SDQ total score (r = −0.63, p < 0.05) within the psychiatric control group. There were no significant correlations between SDQ and tract measurements within the abuse group.
As the correlational analyses were exploratory, we did not correct for multiple comparisons, which would have rendered the findings nonsignificant.
Discussion
To our knowledge, this is the first DTI study to examine the association between documented childhood abuse and alterations in the structure of neural pathways in medication-naive, drug-free youth, controlling for psychiatric comorbidities by the inclusion of a psychiatric control group. This is crucial to elucidate the effects of abuse independently from effects associated with psychiatric comorbidities or medication and drug abuse.10
As hypothesized, the abuse group had significantly reduced white-matter tract volume in the bilateral ILF and left IFoF compared with both healthy and psychiatric controls. At the whole-brain level, the abuse group also had significantly reduced FA in a left-hemispheric posterior region comprising the ILF, IFoF, splenium of the corpus callosum and SLF relative to both healthy and psychiatric controls. Reduced FA of the left IFoF, which was also found in the tractography results, correlated with greater abuse severity in the abuse group. This suggests differences exist not only at the microstructural level as measured by FA, but also at the volumetric level of the entire tract. Thus, differences in the white matter of the ILF and IFoF, particularly in the left hemisphere, was specifically related to the abuse experience. Moreover, reduced FA of the left IFoF was significantly associated with higher SDQ emotion and peer problems in the healthy controls, reinforcing the association between the IFoF and emotional and social behaviours.
The ILF is a ventral associative bundle that mediates the fast transfer of visual signals from the visual areas to the amygdala and hippocampus, and neuromodulatory back-projections from the amygdala to early visual areas, enhancing the visual processing of emotionally significant stimuli.45 It is a key component of the visual–limbic pathway involved in facial affect recognition46 and visual perception.47 The finding of an abuse-specific reduced white-matter microstructure of the ILF extends the findings of earlier studies that found decreased FA of the ILF in adolescents exposed to early neglect23 and in young adults with childhood maltreatment, 26,27 where the decreased FA was furthermore related to poorer visual learning and memory in neglected adolescents23 and with longer duration of abuse.26
The right hemisphere is particularly dominant for negative emotional processing in most individuals.48 Thus, it seems that abuse exposure affects corticolimbic regions involved in emotional regulation and specifically targets the visual–limbic pathway involved in the emotional processing of (aversive) visual information. Given that the abuse experience has both visual and auditory components, the left ILF may also have been compromised as it is involved in language processing.49 Interestingly, studies suggest that fearful facial expressions alone activate the right amygdala, while fearful facial expressions combined with fearful voices activate the left medial temporal gyrus.50 Hence, the combined exposure to fearful faces and voices during a typical severe abuse episode may have disrupted the normal development of both the left and right ILF.
The IFoF, which overlaps spatially and functionally with the ILF, connects the ventral occipital, posterior temporobasal areas to the frontal lobe (inferior frontal, dorsolateral prefrontal and emotion-related OFC regions) and runs parallel to the ILF in its occipital course.51 Hence, it is also involved in facial affect recognition,46 visual and semantic processing, and in multimodal sensorimotor integration.52 Altered microstructure of the IFoF is also consistent with the findings of earlier studies that reported lower FA of the IFoF in adolescents exposed to early neglect23 and in individuals with childhood maltreatment.24,25 The association between abuse experience and microstructure of the IFoF is further underpinned by the present findings of significant negative correlation between abuse severity and FA of the left IFoF.
The splenium of the corpus callosum interconnects the left and right occipital and inferior temporal cortices.51 These regions form the ventral visual stream with reciprocal connections with the hippocampus and emotion-related structures such as the amygdala and OFC.53 The splenium has a protracted myelination trajectory from birth to early adulthood with an accelerated growth during middle childhood that accompanies the development of visual–spatial integration.54 It is involved in the integration of somatosensory and emotional visual information in the 2 hemispheres.55 Our findings also support earlier studies that found reduced FA of the splenium in individuals exposed to childhood maltreatment.24,25
Childhood maltreatment has been associated with abnormal development of the sensory systems that relay adverse sensory experiences. For instance, studies reported structural deficits in the occipital-lingual regions in children with maltreatment56 and psychosocial deprivation,57 in women who experienced childhood sexual/physical abuse,13 and in young adults who witnessed domestic violence during childhood. 14 These findings suggest that the sensory systems that process and interpret adverse sensory inputs may be altered by the abuse experience, reflecting an adaptive response of the developing brain to protect the child from highly hostile environmental conditions by gating sensory experiences and processing related to the abuse.33
Similarly, childhood maltreatment is associated with structural deficits in the emotion-related OFC8–10 and amygdala regions, 58 along with functional abnormalities in frontolimbic regions while processing fearful or angry faces.59,60 Therefore, besides impairment in these individual regions, the findings of white-matter alterations in the ILF and IFoF tracts further suggest disruptions in visual–limbic–OFC pathways mediating sensory integration and cognitive or emotion regulation to sensory stimuli, which may also underlie the neuropsychological deficits in emotion and reward processing61,62 observed in childhood maltreatment.
Given that large-scale epidemiological and longitudinal studies have consistently shown that childhood maltreatment is linked developmentally to psychiatric disorders,29 it is crucial to control for these in order to disentangle the effects of maltreatment from psychiatric comorbidities.10 Therefore, the specificity of the present findings of differences in the ILF and IFoF at both the microstructural and volumetric levels relative to a psychiatric control group in particular extends the findings of previous studies and suggests that these neural pathways are specifically compromised in abused individuals.
The human brain is a highly plastic organ that is continually modified by experience and undergoes changes across the lifespan. The individual neural regions and circuits mature at different rates and have different windows of vulnerability to effects of traumatic stress, with increased vulnerability ascribed to a period of rapid maturation.63 Studies suggest that the maturation of neuronal circuits of the human visual cortex may extend beyond infancy into childhood, with significant development in visual spatial integration between 5 and 14 years of age.54 Given that the ILF, IFoF and splenium show rapid development from childhood with FA increase peaking at early adulthood,64 the visual–limbic pathways may be more susceptible to impairment in individuals with early adversities. Thus, our findings of an association between childhood maltreatment and altered structure of these late developing visual–emotional processing tracts suggests an environmentally triggered disturbance in the normal development of these pathways that may underlie the emotional problems that arise as a consequence of early adversities.
Limitations
Among the strengths of this study are that all participants were medication-naive and drug-free, and their abuse experience was carefully assessed and corroborated by social service records. Also, we included a psychiatric control group to determine the specificity of childhood abuse in our findings. The inclusion of a childhood abuse group without any psychiatric disorders would have provided a more robust means of determining abuse-specific abnormalities; however, such a “pure” group would not be representative of the general childhood abuse populations, as large-scale epidemiological and longitudinal studies have consistently reported that childhood maltreatment is linked developmentally to psychiatric disorders,29 and a meta-analysis further reported a causal relationship between nonsexual childhood maltreatment and a range of mental disorders.65 For the tractography analysis, multiple comparison correction was performed for the number of tracts only and not for the number of diffusion measures, as these are not independent from each other and Bonferroni correction would thus have been too conservative. It is unclear to what extent pubertal development, malnutrition, prenatal drug exposure and presence of current life stressors may have influenced the findings. The moderate sample size of the present study warrants replication in larger samples of youth in future studies. The SES measure used is limited, as it does not provide information on parents’ income and education; however, youth often have difficulties reporting this information.42 Although we recruited participants exposed to childhood physical abuse, it is unrealistic to separate physical abuse from typically co-occurring emotional abuse and neglect; hence, many participants in the abuse group also suffered from emotional abuse and neglect.36
Conclusion
Using medication-naive, drug-free, carefully assessed age- and sex-matched groups of youth exposed to childhood abuse and psychiatric controls matched on psychiatric comorbidities, we found that childhood abuse is associated with altered microstructure of neural pathways connecting the OFC limbic, temporal and occipital visual regions. The abuse-specific abnormalities of the ILF and IFoF visual–limbic pathways may underlie the abnormal emotional regulation to sensory stimuli in victims of abuse.
Acknowledgments
L. Lim and H. Howells were supported by the Reta Lila Weston Trust for Medical Research and the Kids Company UK. L. Lim was also supported by the Lee Kong Chian School of Medicine, Nanyang Technological University Singapore Fellowship Grant (grant number L0491050). H. Howells was supported by the Wellcome Trust (grant number 103759/Z/14/Z). A. Simmons and K. Rubia received research support from the UK Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) for Mental Health at South London and the Maudsley NHS Foundation Trust and Institute of Psychiatry, Psychology and Neuroscience, King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The authors thank all the individuals who participated in this study and their families, as well as the staff of Kids Company London for their help with recruitment.
Footnotes
↵* These authors contributed equally to this work.
Competing interests: M. Mehta has acted as a consultant for Cambridge Cognition and Lundbeck and has received fees from Shire for contribution towards education. K. Mirza has received research and educational grants from GlaxoSmithKline and Shire pharmaceuticals; served on the advisory boards of Janssen, Eli Lilly and Shire pharmaceuticals; and received honoraria for speaking at conferences organized by Janssen, Eli Lilly and Shire pharmaceuticals. K. Rubia has received speaker’s honoraria from Lilly and Shire. No other competing interests declared.
Contributors: L. Lim, H. Hart, M. Mehta, K. Mirza and K. Rubia designed the study. L. Lim, H. Hart and A. Simmons acquired the data, which L. Lim, H. Hart, H. Howells and K. Rubia analyzed. L. Lim and K. Rubia wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received December 12, 2017.
- Revision received September 3, 2018.
- Accepted November 10, 2018.