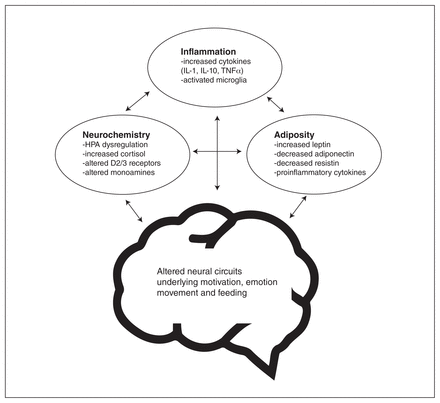
Both obesity and depression are widespread problems with major health and socioeconomic implications. The number of individuals who are overweight or obese has increased dramatically over the last 25 years. Globally, 39% of adults aged 18 and older were overweight and 13% obese,1 and more than 264 million people of all ages suffer from depression.2 Both illnesses are risk factors for a number of chronic diseases, including cardiovascular disease,3,4 and a bidirectional link between risk of depression and obesity in individuals has been proposed.5–7 Given that obesity is on the rise in many countries, an increasingly large proportion of the population is at risk for depression. Vulnerability to depression among people with obesity suggests that there may be mechanistic links underlying these disorders, although the biological mechanisms remain poorly understood. This editorial discusses the evidence for the link between obesity and depression and the neurobiological mechanisms that may underlie this vulnerability (Figure 1). This has the potential to inform clinical evaluation and identify research questions in this area to help further define treatments.
Overview of factors that underlie both obesity and depression and that lead to neural circuit changes. HPA = hypothalamic–pituitary–adrenal axis; IL = interleukin, TNF = tumour necrosis factor.
Epidemiological and observational studies assessing an association between obesity and depression have reported mixed findings. Some studies purport a positive association,8–11 whereas others indicate no association12 or a U-shaped association whereby both underweight and obesity are associated with depression.6 A meta-analysis examining longitudinal studies determined that individuals with obesity were 55% more likely to become depressed, and individuals with depression were 58% more likely to become obese.5 Notably, associations between higher body mass index (BMI) and higher odds of depression are stronger in women than men, with a U-shaped association in men.6,13 Thus, obesity increases the risk of depression and, conversely, depressive disorders are predictive of developing obesity.7 While these studies identify the relative odds of co-occurrence of these disorders, determining if BMI causally influences depression is challenging without a mechanistic understanding of the vulnerabilities.
People with depression and/or anxiety commonly experience a symptom profile that influences appetite, energy and motivation. As a result, presentation of major depressive disorder (MDD) is often consistent with a phenotype that increases vulnerability toward weight gain.14 Individuals with atypical depression may be particularly prone to obesity, as this subtype of MDD is characterized by overeating, oversleeping and fatigue.15 This can be exacerbated by iatrogenic effects of treatment of MDD. The most commonly used atypical antipsychotics, mood stabilizers and antidepressants result in some degree of weight gain.16,17 For example, atypical antipsychotics, such as olanzepine, induce substantial weight gain.18 This can be somewhat mitigated by switching to other medications, such as clozapine; however, this is also known to increase body weight.18 Obesity is also positively associated with anxiety.19 Internalization of negative weight stereotypes may influence this association, as this stigma is associated with negative health consequences.20 For example, obesity is associated with stigma leading to interpersonal distress, which can lead to depression.20 Depression, especially atypical depression, can then result in reduced physical activity, emotional eating, increased alcohol consumption and further development of obesity. While these psychosocial factors underlie the association between obesity and depression, there are several other neurobiological and metabolic factors that may causally underlie vulnerability to these disorders.
Cortisol
The most common biological perturbation associated with depression is an increase in cortisol.21,22 Chronic activation of the hypothalamic–pituitary–adrenal (HPA) axis is associated with chronically stressful or traumatic experiences; immunosuppression; and alteration in monoaminergic pathways, including noradrenaline, dopamine and serotonin.23 Glucocorticoids are normally anabolic, causing increased gluconeogenesis and increased glucose release. High cortisol levels can lead to impaired glucose homeostasis, insulin resistance and visceral fat deposition.24 While these features do not always occur in MDD as they might with Cushing disease,25 patients with depression showed an elevation in cortisol during a social stress test and higher baseline cortisol levels than control participants.26 A possible outcome of higher baseline cortisol is impaired cognitive function. Changes in short-term memory and attention have been reported in individuals with MDD.27–29 Increased cortisol may also underlie increased visceral fat in individuals with depression. In a small sample of women with MDD, there was a 2-times-greater difference in intra-abdominal fat measured with computed tomography compared with the control group matched for body weight, total body fat and BMI.30 This effect was positively correlated with baseline cortisol levels.30 Consistent with this, patients with depression and high cortisol levels have increased visceral fat and insulin resistance compared with those with depression and normal cortisol levels.31 While only about half of patients with depression have elevated cortisol, those with increased cortisol may be susceptible to greater visceral fat deposition and associated metabolic consequences, such as insulin resistance.25 Metyrapone, a cortisol synthesis inhibitor, has shown limited efficacy in patients with treatment-resistant depression.32 However, this broad sample did not specifically identify those with hypercortisolism or those with risk factors for metabolic syndrome. Hair cortisol measurements may be a relatively simple method to identify risk for weight gain in patients with depression and may lead to better implementation of cortisol-lowering therapies.33 Furthermore, use of metformin in individuals with type 2 diabetes to reduce hepatic glucose output and increase insulin sensitivity shows promising effects on improving cognitive function and outcomes in patients with depression and type 2 diabetes.34 Thus, while cortisol-lowering agents or metformin may have limited effects in a broader MDD population, they may be useful for targeting those with subtypes of depression with high cortisol or high risk for metabolic disorders.
Adipokines and inflammation
Another primary neurobiological factor underlying both obesity and depression is increased inflammation. Increased adipocyte size in people with obesity produces local inflammation through increased secretion of cytokines and chemokines.35 Leptin, adiponectin and resistin are termed “adipokines” as they are exclusively released from adipose tissue. Leptin is a cytokine released from adipocytes, and levels circulate in proportion to body fat.36 Leptin acts in the hypothalamus to convey satiety. In people with obesity, leptin receptors can be desensitized, leading to a reduction in negative feedback on both satiety signalling and leptin secretion.36 Leptin resistance also can occur in individuals with atypical MDD, leading to impaired negative feedback and higher leptin levels.15 Leptin treatments in individuals with obesity have generally failed owing to the presence of higher circulating levels of leptin and leptin resistance,37 a fate that could apply if used for treatment of MDD. Adiponectin is another protein released from adipocytes and regulates glucose levels and fatty acid breakdown.38 Adiponectin exerts insulin-sensitizing effects and is inversely associated with obesity and type 2 diabetes.39 Some studies have identified decreased plasma adiponectin associated with MDD,40–42 although a meta-analysis showed no significant differences in adiponectin peripheral levels between individuals with MDD and healthy controls.43 However, they noted that sex and MDD severity were strong moderating factors, whereby women have significantly higher adiponectin levels and the difference in adiponectin between individuals with MDD and controls was positively associated with depression severity.43 Resistin is an adipokine involved in insulin sensitivity and glucose homeostasis.44 While resistin has been implicated in obesity and insulin resistance, it is not yet fully elucidated how resistin plays a role in metabolic dysfunction.44 An exploratory meta-analysis showed that resistin levels are lower in participants with MDD than in healthy controls, although the effect size of this difference was small.43 Adipokines may make promising drug targets for both obesity and MDD. While adipokine antagonists are in preclinical development, human data validating adipokines as novel drugs or drug targets are lacking.45 However, adipokines may prove to be good biomarkers for MDD with an inflammatory or metabolic component, 46 or to help distinguish subtypes of MDD15,47 or MDD from bipolar disorder.48
Proinflammatory cytokines, such as C-reactive protein, tumour necrosis factor–α (TNF-α), interleukin (IL)-1, IL-2 and IL-10, are also increased in individuals with mood disorder symptoms such as anhedonia, depressed mood and lethargy,49 and this is also one of the fundamental characteristics of obesity.50 Obesity status was associated with higher gene expression for inflammatory markers in visceral and subcutaneous adipose tissue, whereas mRNA for inflammatory markers was higher in the visceral adipose tissue of patients who were not obese but not in patients who were obese.51 That study suggests that the visceral adipose tissue may be more sensitive to changes in inflammatory response linked to mood disorders, as has been suggested by others.52 Proinflammatory cytokines released peripherally gain access to the brain through humoural, neural and cellular roots and can influence activation of microglia cells in the hypothalamus and other regions.50 This normally exerts a protective action whereby the immune response activates the HPA axis49 as well as a variety of behaviours, including fatigue, psychomotor slowing, anhedonia and sleep alterations, in efforts to promote reduced exertion during recovery.53 However, chronic activation of microglia and induction of proinflammatory cytokines can have negative effects on neural circuits by influencing plasticity and altering expression of neurotransmitters and their receptors.54 For example, chronic interferon-α treatment downregulates expression of serotonin 1a (5HT1a) and glucocorticoid receptors.55,56 Given that novel immune therapeutics can produce antidepressant effects in individuals with MDD,57 it will be interesting to determine whether this also improves mood outcomes in individuals with obesity.
Peripheral cytokines can potentially infiltrate brain parenchyma through a leaky blood–brain barrier in individuals with depression. In postmortem tissue from humans with depression, there is a decrease in gene expression of CLDN5, for the tight junction protein, claudin 5.58 In the chronic social defeat stress mouse model, targeted suppression of this tight junction protein promoted infiltration of the peripheral cytokine IL-6 into the nucleus accumbens. Decreased blood–brain barrier function was associated with depression-like behaviours in mice,58 but not in stress-resilient mice, because of epigenetic mechanisms that support claudin 5 expression in the blood–brain barrier of the nucleus accumbens.59 Thus, factors that influence blood–brain barrier permeability to peripheral inflammatory cytokines could also be considered as potential biomarkers for MDD.
Dysregulated mesolimbic system
A dysregulated mesolimbic system may also be implicated in obesity and MDD. Mesolimbic dopamine neurons encode the salience or motivational value of rewards.60,61 Individuals with obesity have lower striatal dopamine D2/3 receptor availability62–64 and less striatal responsivity to high calorie beverage taste.65 While decreased D2/3 receptor availability is mainly observed in individuals with severe obesity,62,63,66 increased dopamine with increasing BMI has been observed in individuals with mild obesity,63 suggesting that increased dopamine with BMI may reflect a risk factor for obesity67 and may precede a downregulation of D2/3 receptors with substantial weight gain. Rodent studies indicate that obesity is associated with reduced D2 receptor sensitivity68 and alterations in dopamine turnover in the nucleus accumbens. 69,70 Similarly, anhedonia in rodents is marked by impaired phasic firing of dopaminergic projections to the nucleus accumbens.71 Deep brain stimulation in the nucleus accumbens can reduce anhedonia severity in humans,72 and pramipexole, the high-affinity D2/3 receptor agonist appears to be efficacious at treating individuals with MDD, with prominent anhedonia.73,74 Consistent with this, individuals with depression who had lower D2/3 receptor availability and lower dopamine levels responded best to pramipexole treatment.75 However, while pramipexole has not been tested for efficacy in people with obesity, it has been noted that in treatment of Parkinson disease, pramipexole and other dopaminergic agonists can induce impulse-control disorders, including binge eating,76 limiting their potential efficacy in the treatment of obesity. Thus, both MDD and obesity have associated impairments in dopamine signalling in the mesolimbic circuit. This may influence reward learning and goal-directed behaviour and might have implications for behavioural therapy.
Sleep
Sleep disturbances are also significant in both MDD and obesity. Sleep loss can contribute to the maintenance and/or exacerbation of anxiety and depression.77–79 Notably, disruption of sleep associated with MDD is a significant factor in weight gain.80–82 Several neurobiological factors may underly the association of sleep disruption with weight gain. Reduction in sleep duration decreases the satiety signal, leptin.83–85 A potential mechanism behind decreased leptin release is sleep-restriction-induced increased sympathetic activity,86 an effect known to supress leptin release.87 Sleep disturbances are also associated with increased ghrelin levels.83–85 The enteric hormone ghrelin stimulates appetite and is associated with increased food intake.88 It is possible that increased ghrelin levels during sleep deprivation may be due to increased energy demands during wakefulness.83 Ghrelin synthesis inhibitors, inverse agonists, and receptor antagonists have demonstrated some efficacy in animal models of obesity.89 In contrast, increasing ghrelin induces antidepressant-like responses in chronic social defeat stress in mice.90 However, this has not yet been translated to humans. Sleep disruption also influences glucose homeostasis, resulting in a decrease in insulin sensitivity.91 Chronic elevation of glucocorticoids also leads to insulin resistance through a variety of factors.92 Given that chronic sleep loss can increase HPA activation and cortisol,93,94 this may be another mechanism by which sleep disturbances are associated with increased risk for type 2 diabetes.95 Major depressive disorder is also associated with HPA axis dysregulation, elevated cortisol, disrupted insulin, leptin and ghrelin signalling.96 Thus, it is possible that the metabolic effects associated with sleep disruption may be additive with obesity-induced hormonal dysregulation on risk and outcomes for individuals with MDD. Sleep disturbances are also associated with increased systemic inflammation, an effect that may be activated by adrenergic signalling.97 Given that increased inflammation occurs with obesity and MDD,49,50,54 sleep-loss-induced inflammation may be another moderating factor that underlies the association between obesity and MDD. Suvorexant is a non-benzodiazepine sleep aid that is a competitive antagonist of orexin 1 and 2 receptors. 98 Given that orexin neuropeptides play a central role in the regulation of the sleep–wake cycle, appetite, motivation and affect,99 this treatment strategy may be useful in patients with sleep disruption co-occurring with obesity and depression. Indeed, suvorexant significantly increases sleep efficiency and decreases glucose levels in individuals with insomnia symptoms and type 2 diabetes.100 Furthermore, a similar drug, seltorexant, can improve sleep efficiency and duration in patients with MDD and persistent insomnia who are being treated with antidepressants.101 Future studies should examine if orexin receptor antagonists also decrease inflammation associated with sleep disruption or obesity.
Conclusion
Obesity is a challenging and complex chronic disease. So far, the current tools available for treating obesity are lifestyle modifications and medications, including the lipase inhibitor orlistat (which decreases intestinal fat absorption), phentermine-topiramate and naltrexone-buproprion. Notably, buproprion is a dopamine and norepinephrine reuptake inhibitor antidepressant. Recently, a GLP-1 receptor agonist, semaglutide in combination with behavioural therapy, showed a significant reduction in body weight compared with controls in a double-blind randomized clinical trial.102 This is an encouraging trial, although future assessments should assess if mental health comorbidities are also improved with this treatment. Furthermore, it will be interesting to assess if clinical trials using anti-inflammatories for MDD are effective in weight management. Future trials on existing and novel treatments for obesity should explore the extent to which controlling obesity is sufficient for benefit in individuals with depression. Understanding the link between depression and obesity has not only the potential to inform clinical evaluation, but also to identify novel research questions in this area to help further define treatments. Early identification and management of depression can optimize outcomes and other comorbidities among individuals with obesity. Mental health considerations should be a key factor in behavioural methods of weight control. Furthermore, when initiating pharmacotherapeutic treatment for mental illness, avoid medications with higher metabolic risk. Patients with obesity and comorbid mental illness should be supported with behavioural therapy as part of a multimodal intervention to manage weight.
Acknowledgement
S. Borgland is supported by a Canadian Institutes of Health Research operating grant (CIHR, FDN-147473) and a Canada Research Chair Tier 1 (950-232211).
Footnotes
The views expressed in this editorial are those of the author(s) and do not necessarily reflect the position of the Canadian Medical Association or its subsidiaries, the journal’s editorial board or the Canadian College of Neuropsychopharmacology.
Competing interests: None decalred.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/