Abstract
Background: The present study examined whether mitochondrial DNA copy number (mtDNAcn) and telomere length — key markers of cellular aging — were altered in male and female participants with obsessive–compulsive disorder (OCD) compared to healthy controls. We also tested for associations between these alterations and OCD-related clinical features and inflammatory index.
Methods: A total of 235 patients with OCD (38.7% female) and 234 healthy controls (41.5% female) were included. We quantified whole-blood mtDNAcn and leukocyte telomere length using quantitative polymerase chain reaction. We also calculated the neutrophil-to-lymphocyte ratio from complete blood cell counts.
Results: Multivariate analysis of covariance showed that OCD status had a significant overall effect on cellular aging markers in men (Wilks λ = 0.889, F2,275 = 17.13, p < 0.001) and women (Wilks λ = 0.742, F2,182 = 31.61, p < 0.001) after controlling for age, body mass index and childhood trauma. In post-hoc comparisons, men with OCD had lower mtDNAcn than controls (p < 0.001), but we found no between-group difference for telomere length (p = 0.55). Women with OCD had a significantly lower mtDNAcn (p < 0.001) and shortened telomere length (p = 0.023) compared to controls. Moreover, the lower mtDNAcn shown in the OCD group was significantly correlated with an increase in systemic inflammation for both sexes, as measured by neutrophil-to-lymphocyte ratio.
Limitations: The present cross-sectional design did not allow us to infer a causal relationship between OCD disease status and cellular aging markers.
Conclusion: The present study is, to our knowledge, the first to demonstrate alterations in mtDNAcn and telomere shortening in OCD. These results suggest that aging-associated molecular mechanisms may be important in the pathophysiology of OCD.
Introduction
Obsessive–compulsive disorder (OCD) is a chronic neuropsychiatric disorder characterized by repetitive, intrusive and time-consuming thoughts (obsessions) and irresistible irrational behaviours or rituals (compulsions), which can cause severe distress to the patient.1 People with OCD often identify traumatic events and psychosocial stress as factors that develop and exacerbate their symptoms.2 In addition, cross-sectional case–control studies have shown oxidative imbalance3,4 and oxidative DNA damage5 in patients with OCD, suggesting that oxidative stress may play a crucial role in the pathophysiology of OCD. Although the effects of stress on the pathophysiology of OCD remain unclear, chronic stress may influence the underlying biochemical processes and neurobiological changes that occur in OCD.
Recently, the association of cellular aging with chronic stress and neuropsychiatric conditions has become a growing field of research. People with serious mental illnesses are prone to various physical health problems.6 Physical morbidities such as cardiovascular and metabolic diseases were more prevalent in patients with psychiatric disorders than in the general population.7 Recent research has shown that OCD is associated with an increased risk of allergies and endocrine diseases.8,9 Such comorbidity for physical diseases and psychiatric disorders may be associated with accelerated cellular aging.10 However, few studies have investigated the role of cellular aging in OCD.
Accumulating evidence has shown that the shortening of telomere length in response to cumulative oxidative stress11,12 is implicated in accelerated cellular aging and increased physical morbidity in neuropsychiatric conditions.13,14 As well, mitochondrial function has attracted much attention as a biomarker of stress adaptation and biological aging in chronic stress and psychiatric disorders.15 Mitochondria — highly dynamic organelles located in the cytosol of eukaryotic cells — play a key role in cellular energy production and homeostasis by producing adenosine triphosphate through oxidative phosphorylation, and their fission and fusion processes help to facilitate adaptation to the changing environment in response to metabolic demand or stress.16 Altered mitochondrial DNA copy number (mtDNAcn), an index of mitochondrial dysfunction, has been associated with several psychiatric disorders, including posttraumatic stress disorder17 and bipolar disorder,18 although there have also been mixed findings of higher, reduced or unchanged mtDNAcn in psychiatric disorders. To date, no study has investigated whether telomere shortening or altered mtDNAcn occur in OCD.
In the present study, we examined whether leukocyte telomere length and mtDNAcn were altered in male and female participants with OCD compared to healthy controls. We also tested whether these markers were associated with clinical and inflammatory factors of OCD. Given the role of stress and the possible involvement of oxidative stress in the pathogenesis of OCD, we hypothesized that participants with OCD would exhibit telomere shortening and altered mtDNAcn compared to healthy controls, and that the alterations would be associated with clinical features and an inflammatory index of OCD.
Methods
Participants
A total of 243 patients with OCD (94 women) and 241 healthy controls (99 women) were included in the present study. The patients with OCD were recruited at an OCD-specialized outpatient clinic at Severance Hospital, Yonsei University Health System (Seoul, Republic of Korea). Healthy controls were recruited through advertisements. All participants were assessed by trained psychiatrists using the Structured Clinical Interview for the DSM-IV19 to evaluate them for the presence of current and previous psychiatric disorders. To be included in the OCD group, people had to be aged 19 to 50 years and have a primary diagnosis of OCD. A psychiatrist (S.J. Kim) confirmed the diagnosis based on the DSM-IV.19 Exclusion criteria were psychosis, bipolar disorder, substance dependence, mental retardation as defined by the DSM-IV, head trauma, major somatic and neurologic disorders, pregnancy and breastfeeding. The ethnicity of all participants was self-reported and only those who were ethnically Korean were enrolled. All participants provided written informed consent before participation, and this study was approved by the institutional review board of Severance Hospital, Yonsei University Health System.
Measurement of telomere length and mtDNAcn in blood
Genomic DNA was isolated from peripheral blood samples using a nucleic acid isolation device, the QuickGene-mini80 (Fujifilm). Telomere length and mtDNAcn were then measured in the Blackburn Laboratory at the University of California, San Francisco. Telomere length (relative telomere to single copy gene ratio) was measured using quantitative polymerase chain reaction (qPCR) based on a method originally published by Cawthon.20 DNA samples were assayed twice in triplicate wells. If the difference between the 2 runs was greater than 7%, a third run was conducted and the average of the closest values was reported. The inter-assay coefficient of variation for telomere length was 2.1%. A detailed description of the assay procedures has been described previously.21
For mtDNAcn, the relative copy number of mtDNA per diploid nuclear genome was determined using a TaqMan multiplex assay. This involved the detection of a 69 bp fragment of the ND1 gene in mtDNA (nucleotides 3485–3553) and an 87 bp fragment of RNase P (TaqMan Copy Number Reference Assay, human, RNase P; cat. no. 4403328; Life Technologies) in the nuclear genomic DNA, based on a previously published method.22 DNA samples were assayed in triplicate wells in a single run. A subset of samples was randomly selected for a second run to obtain the inter-assay coefficient of variation, which was 4.1% for mtDNA. Details of the assay procedures have been previously described.17 All qPCR tests were performed using the LightCycler 480 Instrument II (Roche Diagnostics).
Measures of clinical characteristics
The clinical symptoms of OCD were evaluated by a trained psychologist using the 10-item Yale–Brown Obsessive Compulsive Scale23 for obsessive–compulsive symptom severity and the Montgomery–Åsberg Depression Rating Scale24 for depressive symptoms.
Age at onset of OCD was considered to be the age at which obsessive or compulsive symptoms first occurred as remembered by patients or family members and was dichotomized as early-onset (≤ 17 years) and late-onset (> 17 years).25
Childhood traumatic experiences were assessed using the Early Trauma Inventory–Self-Report Short Form,26 which consists of 27 items in 4 different domains of childhood traumatic experiences: general trauma and physical, sexual and emotional abuse. The scores for all 4 domains are summed.
Measure of neutrophil-to-lymphocyte ratio as an inflammatory marker
We analyzed the complete blood count with differential white blood cell count in a subset of the participants with OCD (n = 202) using the XN-20 (Sysmex). We calculated the neutrophil-to-lymphocyte ratio (NLR), an easily measured marker associated with systemic inflammation,27 from the complete blood count. Participants with a white blood cell count greater than 10 000 cells/μL (n = 18) or those with extreme NLR outliers (n = 2) were excluded from our analyses.
Statistical analyses
We compared demographic and clinical characteristics between groups using independent-samples t tests for continuous variables and Pearson χ2 tests for categorical variables. We calculated Pearson correlation (r) and partial correlation (pr) coefficients to examine relationships between the cellular markers and other variables. We performed multivariate analysis of covariance (MANCOVA) and post-hoc comparisons using the 2 cellular markers as dependent variables and OCD status as an independent variable. Because lifespan and aging-related markers such as telomere length or attrition rate are affected by sex differences,28 we performed MANCOVAs in sex-stratified analyses to control for the possible effect of sex difference and reduce potential bias related to unmeasured interaction effects between sex and clinical features. In the partial correlation analysis and MANCOVAs, we controlled for potential confounders (age, body mass index and childhood trauma) that have been associated with cellular aging and oxidative stress.29,30 As well, to examine the relationships between the 2 markers (telomere length and mtDNAcn) and clinical characteristics of OCD, we conducted hierarchical multiple linear regression analyses with 2 steps in each sex. In these analyses, we entered age, body mass index and childhood trauma as a block in the first step, and then 4 clinical characteristics (age at onset, duration of illness, Yale–Brown Obsessive Compulsive Scale score and Montgomery–Åsberg Depression Rating Scale score) as a second block in the next step. We analyzed all data using the Statistical Package for the Social Sciences, version 25.0 (SPSS Inc.). All tests were 2-tailed, and p < 0.025 was considered statistically significant based on Bonferroni correction for 2 dependent variables. Data values are presented as mean ± standard deviation.
Results
Sample characteristics
Of those who participated in the interview (243 patients with OCD and 241 controls), data from 8 patients and 7 controls were excluded from the final analysis: 7 controls had comorbid psychiatric disorders, 5 patients with OCD had incomplete self-reported data, 2 samples contained insufficient DNA, and 1 patient had an outlier telomere length value. The final analyses included 235 patients with OCD (91 women) and 234 healthy controls (97 women).
The mean ages for the OCD and control groups were 27.70 ± 7.55 years and 27.23 ± 7.72 years, respectively. We found no significant differences between the groups with respect to age, sex, education status or body mass index. Based on Early Trauma Inventory total scores, those in the OCD group had experienced more childhood trauma than those in the control group (p < 0.001; Table 1).
Demographic and clinical characteristics of patients with OCD and healthy controls
Demographic and psychosocial factors associated with telomere length and mtDNAcn
In correlation analyses for the entire study population, age was inversely correlated with telomere length (r = −0.303, p < 0.001), but was not significantly correlated with mtDNAcn (r = −0.006, p = 0.89). Childhood trauma based on Early Trauma Inventory total score had a weak negative correlation with mtDNAcn (r = −0.094, p = 0.04) but not telomere length (r = −0.031, p = 0.50). As well, mtDNAcn was positively associated with telomere length (r = 0.179, p < 0.001). When controlling for age, body mass index and childhood trauma, the correlation between mtDNAcn and telomere length remained significant for the entire study population (pr = 0.182, p < 0.001) and for the control and OCD groups (healthy controls pr = 0.153, p = 0.020; patients with OCD pr = 0.206, p = 0.002).
Among healthy controls, women exhibited higher telomere length (women 1.099 ± 0.225 v. men 1.043 ± 0.225, p = 0.04) and higher mtDNAcn (women 551.7 ± 103.1 v. men 519.5 ± 119.5, p = 0.03) than men. Among patients with OCD, neither telomere length (women 1.030 ± 0.184 v. men 1.050 ± 0.225, p = 0.43) nor mtDNAcn (women 423.6 ± 124.5 v. men: 434.3 ± 116.2, p = 0.51) was significantly different between women and men.
MANCOVA
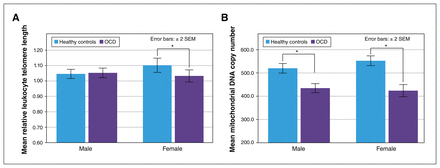
MANCOVA using age, body mass index and childhood trauma as covariates showed that OCD status had a significant overall effect on mtDNAcn and relative telomere length in both men (Wilks λ = 0.889, F2,275 = 17.13, p < 0.001) and women (Wilks λ = 0.742, F2,182 = 31.61, p < 0.001; Table 2). In post-hoc comparisons, male participants with OCD had a significantly reduced mtDNAcn compared to healthy controls (p < 0.001), but we found no significant difference in relative telomere length between the 2 groups (p = 0.55). In contrast, female participants with OCD had a significantly reduced mtDNAcn (p < 0.001) and a shortened telomere length (p = 0.023) compared to healthy controls (Table 2 and Figure 1).
Relative leukocyte telomere length and whole blood mitochondrial DNA copy number for male and female patients with OCD and healthy controls. *p < 0.05. Error bars represent ± 2 standard errors of the mean. OCD = obsessive–compulsive disorder; SEM = standard error of the mean.
MANCOVA of leukocyte telomere length and mitochondrial DNA copy number data from male and female OCD and control groups
Medication in patients with OCD
Of the 144 men with OCD, 45 (31.3%) were drug-naive or had been drug-free for more than 8 weeks; the other 99 (68.7%) had been prescribed serotonin reuptake inhibitors. Of the 91 women with OCD, 32 (35.2%) were drug-naive or drug-free for more than 8 weeks, and 59 (64.8%) were taking serotonin reuptake inhibitors. We found no significant difference between unmedicated and medicated patients with respect to telomere length (women 1.064 ± 0.204 v. 1.012 ± 0.172, p = 0.20; men 1.072 ± 0.193 v. 1.039 ± 0.181, p = 0.33) or mtDNAcn (women 446.3 ± 120.6 v. 411.3 ± 125.9, p = 0.20; men 430.5 ± 111.0 v. 436.0 ± 119.0, p = 0.33) in either sex.
Association between mtDNAcn and telomere length and clinical characteristics of OCD
Hierarchical multiple linear regression analyses showed no significant associations between mtDNAcn or telomere length and symptom severity of OCD, depressive symptoms, age at onset or duration of illness in participants with OCD of either sex.
Relationship of an inflammatory factor (NLR) with mtDNAcn and telomere length in men and women with OCD
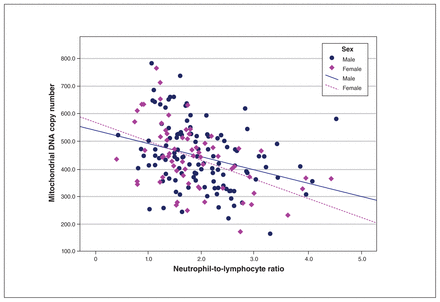
In partial correlation analyses with covariates of age, body mass index and childhood trauma, we found a significant association between NLR and mtDNAcn in both men and women with OCD (men [n = 115] pr = −0.330, p < 0.001; women [n = 67] pr = −0.519, p < 0.001). Figure 2 shows a scatterplot of raw scores for the 2 variables, which were inversely correlated in each sex. We found no significant association between NLR and telomere length for either sex.
Association between neutrophil-to-lymphocyte ratio and mitochondrial DNA copy number. Using original raw data without adjusting for covariates, the scatterplot shows an inverse relationship for both sexes.
Discussion
In the present study, we investigated whether leukocyte telomere length and whole-blood mtDNAcn were associated with OCD in a relatively homogeneous Korean population. To the best of our knowledge, this was the first study to investigate the role of telomere length and mtDNAcn in the pathophysiology of OCD. We demonstrated significantly reduced telomere length and mtDNAcn in female participants with OCD compared to healthy controls. Male participants with OCD also had a significantly reduced mtDNAcn compared to healthy controls, but we found no significant difference in telomere length between the 2 groups. These results suggest the possible involvement of mitochondrial dysfunction and telomere erosion in the pathophysiology of OCD.
We demonstrated a reduction in mtDNAcn in both male and female participants with OCD. The mtDNAcn in the present OCD sample showed no significant associations with clinical variables such as duration of illness or Yale–Brown Obsessive Compulsive Scale, possibly because of a lack of statistical power to detect an association; another possible explanation is that mtDNAcn may be a predisposing factor for OCD rather than a state factor of clinical features. Although little is known about whether OCD is associated with altered mtDNAcn, there has been indirect evidence of mitochondrial involvement in OCD. Occurrence of OCD has been reported in cases with mitochondrial disease.31 In addition, genetic variants associated with mitochondrial disease have been identified in patients with OCD.32 Although the underlying mechanisms linking mitochondrial dysfunction to OCD are unclear, a growing body of evidence suggests that the role of mitochondria in cellular stress responses and regulation of energy metabolism and homeostasis may be important in pathophysiological changes of the brain to stress.33 Notably, mitochondria are principal hubs in the regulation of immune and inflammatory responses.34 A study in Taiwanese, community-dwelling older adults showed that low mtDNAcn was associated with high inflammatory markers.35 Similarly, the reduction in mtDNAcn shown in the OCD group from the present study was significantly correlated with an increase in systemic inflammation as measured by the NLR. It has been suggested that cumulative damage to mitochondria and mtDNA induced by reactive oxygen species affects inflammatory responses and cellular senescence.36 In turn, inflammatory stress leads to enhanced reactive oxygen species production and cumulative damage to mtDNA, and the interplay between oxidative stress and mitochondrial dysfunction can result in energy failure, biological aging and the pathogenesis of age-related diseases.37,38 Thus, interplay between reduced mtDNAcn and elevated inflammation might contribute to the development of OCD.
On the other hand, mtDNAcn was not correlated with age in our sample, which consisted mostly of young adults. Although there has been some evidence showing an age-related decline in mtDNAcn,39–41 several reports (including our findings) have found no association between mtDNAcn and age.42–44 This inconsistency in findings may be because of different age groups used and a narrow age range among participants. Findings from previous literature of age-related decline in mtDNAcn was observed for participants older than 50 years.39,45 In addition, although mtDNAcn quantification has been used as a biomarker for mitochondrial function and has been implicated in age-related diseases,46 it is not a direct measure of the functionality of individual mitochondria and may reflect oxidative stress states rather than cellular aging itself.44,47 Indeed, mtDNAcn as measured by qPCR, which reflects the relative quantity of mitochondrial DNA compared to the nuclear DNA in a sample, should be considered a proxy for the content of mitochondrial copies. Mixed findings have been also reported with respect to the direction of altered mtDNAcn in several neuropsychiatric disorders. One study showed a significant reduction in mtDNAcn in combat-related posttraumatic stress disorder and an “inverted-U” relationship between mtDNAcn and symptom severity of posttraumatic stress disorder.17 In contrast, increased mtDNAcn has been reported in patients with attention-deficit/hyperactivity disorder48 and major depression,49 and was associated with childhood trauma and lifetime psychopathology,50 suggesting that an increase in mtDNAcn might be a compensatory cellular response to mitochondrial dysfunction.51 Interestingly, a meta-analysis of mtDNAcn studies in bipolar disorder revealed no association between bipolar disorder and alterations in mtDNAcn, but Asian populations with bipolar disorder showed significantly reduced mtDNAcn compared to controls.18 This observed discrepancy in results might be related to differences among studies in methods for extracting DNA and measuring mtDNAcn. Further research using a reliable measure of mitochondrial function and oxidative stress is needed to determine the involvement of mitochondrial dysfunction and cellular aging in neuropsychiatric diseases such as OCD.
After controlling for age, body mass index and childhood trauma, female participants with OCD exhibited significantly shorter telomere length compared to healthy controls. Although there is little direct evidence of altered telomere length in OCD, some indirect evidence supports an association between telomere shortening and OCD. Increased oxidative stress and oxidative imbalance have been implicated in the pathophysiology of OCD,32,52,53 and it is well established that oxidative stress is associated with shortened telomere length.14 Furthermore, increased oxidative DNA damage has been noted in OCD,54 which is known to induce telomere attrition.55 In this study, we observed telomere shortening in female, but not male, patients with OCD, possibly because women are more vulnerable to OCD-associated telomere erosion or because of potential differences in clinical features between men and women. Because the findings from sex-stratified analyses cannot clarify the contribution of sex to the cellular aging process, future research is needed to help determine the role of sex in telomere biology and mitochondrial function, and their different associations with psychiatric disorders.
Although the mechanisms linking cellular aging markers to OCD remain to be elucidated, it is unlikely that mitochondrial dysfunction and telomere erosion affect OCD pathogenesis via independent pathways. There is emerging evidence that these are linked biologically and have a complicated telomere–mitochondrial relationship during the human aging process.56 Consistent with the findings of this study, leukocyte telomere shortening and mtDNAcn have been positively intercorrelated in healthy individuals57,58 and in pregnant women,59 suggesting coregulation of telomere biology and mitochondrial function.58 Telomere dysfunction is known to reciprocally affect altered mitochondrial biogenesis.60 In addition, both oxidative stress and telomere erosion may be induced by mitochondrial dysfunction in response to stress.61 Although the underlying mechanisms are not well understood, elevated stress and proinflammatory cytokines may induce both mitochondrial dysfunction and altered telomere biology.15 Further research elucidating the complicated telomere–mitochondrial relationship and its underlying mechanisms is required.
Limitations
In the present study, we measured mtDNAcn and telomere length in peripheral blood, and not in brain tissue. It is unclear whether peripheral biomarkers reflect brain status in neuropsychiatric disorders. Notably, a postmortem study found that people who had died by suicide had a higher mtDNAcn in peripheral blood but a lower mtDNAcn in the prefrontal cortex compared to controls.62 Shorter telomere length was also identified in both the peripheral blood and prefrontal cortex of the same people, showing an opposite relationship between telomere length and mtDNAcn in postmortem blood and brain tissue. However, whether these peripheral biomarkers accurately reflect neuronal biomarkers remains unclear, and this is a methodological limitation of human research involving clinical populations.
The present study had other limitations. First, the cross-sectional design did not allow us to infer a causal relationship between OCD disease status and cellular aging markers. Second, we controlled for the possible effects of age, childhood trauma and body mass index, but did not consider other potential confounders that may affect oxidative stress, such as recent stressors, smoking, exercise and nutrition. Third, we did not assess other potentially related biomarkers such as oxidative stress markers and telomerase activity. Fourth, we measured only cellular mtDNAcn in leukocytes and did not detect circulating mtDNA levels in cell-free plasma, which may be released into the circulation by the mitochondria under stress.63 In addition, although we found no significant relationship between platelet counts and mtDNAcn among participants with OCD (data not shown), mtDNAcn measured using the extracted genomic DNA from whole blood can be affected by mtDNA in platelets.64 Finally, although the results of the present study did not show a significant effect of medication status on mtDNAcn or telomere length, psychotropic drug treatments may affect cellular aging. The influence of psychotropic medications on mitochondrial processes has been investigated mainly using animal studies and thus remains unclear.65 Future longitudinal studies including larger samples of drug-naive patients with OCD are needed to confirm the present findings and elucidate the role of mitochondrial dysfunction in the pathophysiology of OCD.
Conclusion
The present study is the first to demonstrate alterations in mtDNAcn in OCD. We also identified telomere shortening in women with OCD. Our results suggest that aging-associated molecular mechanisms may be important in the pathophysiology of OCD. Future studies involving larger cohorts are warranted to investigate the role of mitochondrial dysfunction and telomere length in OCD and elucidate the molecular mechanisms that underlie OCD-related pathophysiology.
Footnotes
Funding: This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2018R1A2B2007714 and NRF-2019R1A2C1084611). The funding source did not influence the study design, data collection, analysis and interpretation of data, writing of the report or the decision to submit the article for publication.
Competing interests: None declared.
Contributors: J.-I. Kang and S.-J. Kim designed the study. C.-I. Park and S.-J. Kim acquired the data, which J. Lin, S.-T. Kim, H.-W. Kim and S.-J. Kim analyzed. J.-I. Kang wrote the article, which C.-I. Park, J. Lin, S.-T. Kim, H.-W. Kim and S.-J. Kim reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received December 23, 2020.
- Revision received February 26, 2021.
- Accepted March 17, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/