Abstract
Background: Deep brain stimulation (DBS) is a promising investigational approach for treatment-resistant depression. However, reports suggesting changes in personality with DBS for movement disorders have raised clinical and ethical concerns. We prospectively examined changes in personality dimensions and antidepressant response to subcallosal cingulate (SCC)-DBS for treatment-resistant depression.
Methods: Twenty-two patients with treatment-resistant depression underwent SCC-DBS. We used the NEO Five-Factor Inventory for personality assessment at baseline and every 3 months until 15 months post-DBS. We assessed depression severity monthly using the Hamilton Depression Rating Scale.
Results: We found a significant decrease in neuroticism (p = 0.002) and an increase in extraversion (p = 0.001) over time, showing a change toward normative data. Improvement on the Hamilton Depression Rating Scale was correlated with decreases in neuroticism at 6 months (p = 0.001) and 12 months (p < 0.001), and with an increase in extraversion at 12 months (p = 0.01). Changes on the Hamilton Depression Rating Scale over time had a significant covariate effect on neuroticism (p < 0.001) and extraversion (p = 0.001). Baseline openness and agreeableness predicted response to DBS at 6 (p = 0.006) and 12 months (p = 0.004), respectively.
Limitations: Limitations included a small sample size, a lack of sham control and the use of subjective personality evaluation.
Conclusion: We observed positive personality changes following SCC-DBS, with reduced neuroticism and increased extraversion related to clinical improvement in depression, suggesting a state effect. As well, pretreatment levels of openness and agreeableness may have predicted subsequent response to DBS. The NEO Five-Factor Inventory assessment may have a role in clinical decision-making and prognostic evaluation in patients with treatment-resistant depression who undergo SCC-DBS.
Introduction
Treatment-resistant depression is a disabling, life-threatening disorder. Approximately 33% of people with depression do not respond to 4 trials of antidepressants,1 and 20% fail to respond to electroconvulsive therapy.2 Treatment-resistant depression leads to decreased productivity, multiple hospitalizations and financial burden for health systems.3 The estimated incidence of completed suicide in people with treatment-resistant depression is twice that in people with nonresistant depression.4
Deep brain stimulation (DBS) is a promising experimental treatment that can be applied to several brain targets for advanced treatment-resistant depression.5 The key advantages of DBS over ablative surgery include its reversibility and the adjustability of stimulation. It is also much less burdensome than other maintenance treatments for treatment-resistant depression, such as electroconvulsive therapy, transcranial magnetic stimulation or intravenous ketamine. The most common DBS target for treatment-resistant depression is the subcallosal cingulate (SCC) region. Several open-label trials of SCC-DBS have reported response rates of 40%–60%, which is substantial given the morbidity and mortality associated with treatment-resistant depression.5 We recently reported response rates of 45%–50% in 22 patients with treatment-resistant depression after 6 and 12 months of SCCDBS.6 In addition to being effective, SCC-DBS is also safe, with limited transient adverse effects.5
In recent years, DBS-induced personality changes have become a hotly discussed and debated clinical and ethical issue, particularly in patients with Parkinson disease who receive DBS in the subthalamic nucleus. Several studies have reported changes in personality, especially in mood and behaviour, following DBS in the subthalamic nucleus,7–9 some of which were harmful and disruptive to working life and sociofamilial relations. Because DBS of brain targets for psychiatric conditions could alter mood, behaviour and personality, a few recent studies have investigated the relationship between DBS and personality changes in patients with mental disorders. Bewernick and colleagues10 reported an absence of quantitative change in personality, as measured by the Neuroticism–Extraversion–Openness Five-Factor Inventory (NEO-FFI),11 with DBS in the superolateral medial forebrain bundle (slMFB) in patients with treatment-resistant depression over a period of 5 years. Another study reported positive changes in self-experience related to reduced compulsions in 72% of patients with obsessive–compulsive disorder who received DBS of the anterior limb of the internal capsule.12 However, the findings of these studies could not be generalized to other brain targets or patient populations.
The assessment of personality-related changes with DBS remains challenging because of the complexities of defining personality constructs and the lack of reliable or sensitive measurements.13 Personality is described as the organized set of a person’s characteristics that uniquely influence his or her cognition, motivations, feelings and behaviour in various situations.14 The most influential model of human personality is the 5-factor model (Big 5), which describes personality in terms of 5 dimensions: neuroticism, extraversion, conscientiousness, agreeableness and openness to experience.11 The NEO inventory is a widely used, reliable, validated instrument for the assessment of the Big 5 personality dimensions in depression. Among these Big 5 dimensions, neuroticism and extraversion are studied frequently, because they represent vulnerability to mood and anxiety disorders and are subject to change with current mood state (state effect)15,16 and antidepressant treatment (treatment effect).17,18 Altered neuroticism following SCC-DBS may be particularly likely, because this brain region is involved in the neural processing of negative emotions such as sadness and fear.19,20 Neuroticism is associated with a tendency to experience negative emotion such as anxiety or fear, sadness, anger and disgust.11 Hass and colleagues found that neuroticism was associated with higher SCC activation during trials of high emotional conflict than in trials of low emotional conflict,21 and during fear processing in a serotonin-depletion condition.22 Neuroticism and increased SCC activation in emotional-task-dependent functional MRI were strongly associated with the severity of depressive symptoms,23,24 and pretreatment abnormalities of high neuroticism and increased SCC metabolic activity associated with depression are subject to change with antidepressant treatments.25,26 In contrast to neuroticism, extraversion is associated with a tendency to experience positive affect and is linked with neural systems responsible for sensitivity to reward and neural processing of pleasure involving the nucleus accumbens and the orbitofrontal cortex.27 The SCC also has strong anatomic connections to the nucleus accumbens and the MFB,28–30 and it plays a critical role in selective aspects of reward-processing.31 Decreased extraversion was associated with depression state, and increased extraversion was associated with depression recovery,15 suggesting that clinical improvement may affect extraversion.
This study set out to determine whether SCC-DBS for treatment-resistant depression was associated with personality changes over time, whether personality changes were dependent on or independent of improvement in depression, and whether personality dimensions would predict response to DBS. Based on the known effect of antidepressant treatments on neuroticism and extraversion and the link between the SCC, neuroticism and the reward pathway, we predicted a decrease in neuroticism and an increase in extraversion following SCC-DBS, and that these changes would be associated with clinical improvement for treatment-resistant depression.
Methods
Patients
Twenty-two participants (12 male, 10 female) were recruited and underwent SCC-DBS at the Foothills Medical Centre in Calgary, Alberta, Canada. Details on the trial design, patient selection (inclusion and exclusion criterion), methods and surgical protocol of this study have been reported elsewhere.6 In short, all eligible participants were aged 20–70 years and had a current diagnosis of major depressive disorder or bipolar depression as defined by DSM-IV, with a score on the 17-item Hamilton Depression Rating Scale (HDRS)32 greater than or equal to 20 at the time of recruitment. All participants demonstrated treatment resistance.6 Exclusion criteria were other Axis I disorders or severe personality disorders, previous neurologic injury, neurodegenerative disorders, pregnancy, and medical and general contraindications for DBS surgery. The University of Calgary Ethics Review Board approved the study, and all patients provided informed consent (registered at ClinicalTrials.gov NCT01983904).
Patients received bilateral SCC-DBS stimulation in a double-blind, randomized, controlled crossover trial. Patients were randomized to 1 of 2 stimulation types: long pulse width (210–450 μs) and low voltage stimulation (4 V), or short pulse width (90 μs) and high voltage stimulation (4–8 V), keeping the frequency constant (130 Hz).6 The primary clinical outcome measure was the clinician-rated 17-item HDRS, completed at baseline and every month for 15 months. Patients who had not responded to DBS (< 50% reduction in HDRS score from baseline) at 6 months were crossed over to the opposite stimulation arm for another 6 months. Responders (≥ 50% reduction in HDRS score from baseline) continued with the same stimulation for another 6 months. All patients received add-on individual cognitive behavioural therapy. Responders (n = 9) at 6 months received cognitive behavioural therapy from 6 to 9 months following DBS, and the rest (n = 12) received it from 12 to 15 months following DBS.
Personality assessment
Personality was measured using the NEO-FFI.11 This is a self-report measure that uses a 5-point Likert scale to score 5 dimensions of personality: neuroticism, extraversion, openness, agreeableness and conscientiousness. The NEO-FFI is a reliable instrument with adequate internal and temporal reliability.33 Personality was assessed at baseline (pre-DBS) and every 3 months up to 15 months. Patients were also asked whether they perceived any change in their personality (changes in feelings, thoughts or behaviour) in a semistructured interview at their last follow-up, between 36 and 64 months (Appendix 1, Table S1, available at jpn.ca/210028-1-at).
Statistical analysis
We determined sample size for the main clinical trial based on the primary clinical outcome measure. Because the present study used the secondary outcome measure of personality changes, this analysis should be considered exploratory. We completed statistical analyses using SPSS Statistics version 25.0 (IBM). We used χ2 tests and independent-sample t tests to look for differences in demographics and clinical characteristics between DBS responders and nonresponders. We used 1-sample t tests to compare the sample mean to the normative mean. We used a linear mixed model analysis to examine personality scores over 15 months. We chose a linear mixed model because of missing data. To examine the main effect of time in the primary model, we constructed 5 separate linear mixed model models for the 5 personality dimensions (neuroticism, extraversion, conscientiousness, openness, agreeableness) as dependent variables, using time as the fixed effect at 6 levels (baseline and 3, 6, 9, 12 and 15 months) and participants as random effects and as the intercept. We used a Bonferroni confidence interval adjustment for post hoc pair-wise comparisons. We used Pearson correlations to determine the relationship between change in depression scores and change in personality scores. To further assess the effect of depression on personality change over time, we created a separate linear mixed model using time and the interaction of time and depression scores as fixed effects and personality dimensions that changed significantly with time in the primary model as dependent variables. We used independent-sample t tests to look for baseline personality predictors between treatment responders and nonresponders at 6 and 12 months. We analyzed potential confounds for covariate effects on baseline personality predictors. We examined the effect of cognitive behavioural therapy on personality scores using paired t tests for before and after add-on cognitive behavioural therapy. Results were considered statistically significant at p ≤ 0.01, because of Bonferroni correction for the 5 personality dimensions.
Results
Participant characteristics
Participant demographic and clinical characteristics are summarized in Table 1. The baseline (mean ± standard deviation [SD]) HDRS score was 23.73 ± 3.86. Among 22 patients with treatment-resistant depression, 17 were diagnosed with major depressive disorder and 5 were diagnosed with bipolar depression. Ten patients (45%) were randomly assigned to long pulse-width stimulation and 12 (55%) to short pulse-width stimulation.
Demographic and clinical characteristics of participants
Clinical outcomes
Of the full sample, 10 were responders to DBS treatment (45%) and 12 were nonresponders (55%) at 6 months post-DBS, as per the intention-to-treat analysis. One responder who died by suicide in the third month was excluded; the analysis for this study was conducted based on observed cases per protocol. As previously reported,6 responders (n = 9) at 6 months were significantly younger than nonresponders (t19 = −2.673, p = 0.015; Table 1). Responders (n = 10) at 12 months were not significantly different from nonresponders (n = 11) with respect to any baseline clinical or demographic characteristics (Table 1). Response rates were comparable between the long pulse-width and short pulse-width stimulation groups. At 6 months and 12 months, 40% and 54% were responders in the long pulse-width group, respectively, and 50% and 45% were responders in the short pulse-width group.6 We did not analyze responders and nonresponders from each stimulation group separately because of the small sample size.
Baseline personality profiles compared with normative data
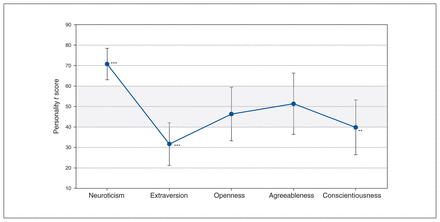
We compared participants’ personality-profile t scores with a normative sample33 (mean ± SD, 50 ± 10; Figure 1). Baseline personality scores were not available for 1 patient, who was excluded from the cross-sectional baseline analysis. Neuroticism was significantly higher in the study sample than in the normative sample (t20 = 12.382, p < 0.001), and both extraversion (t20 = −8.056, p < 0.001) and conscientiousness (t20 = −3.483, p = 0.002) were significantly lower in the study sample. The study sample did not differ significantly from the normative sample in terms of openness (t20 = −1.283, p = 0.21) or agreeableness (t20 = 0.418, p = 0.68). The significantly higher scores in neuroticism and lower scores in extraversion were considered clinically meaningful, because they all exceeded 1 SD from the normative mean. However, patient scores for conscientiousness were within the range of 1 SD, and therefore were not clinically meaningful.
Personality dimensions at baseline compared to normative data. Patients with treatment-resistant depression (n = 21) compared to the healthy normative sample (mean ± standard deviation) on 5 personality dimensions. Grey shaded area represents published normative data. ***p < 0.001 and clinically meaningful (> 1 standard deviation; 1 standard deviation = 10 points on the scale); **p < 0.01. Neuroticism Extraversion Openness Agreeableness Conscientiousness
Personality scores over time
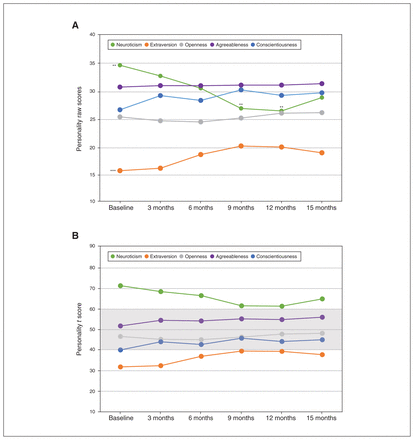
Descriptive statistics, including the mean ± SD of scores for each personality dimension at each time point, are given in Appendix 1, Table S1. Neuroticism decreased significantly over time (F5,18.47 = 5.876, p = 0.002), specifically from baseline to 9 months (p = 0.002) and baseline to 12 months (p = 0.002; Figure 2A). Extraversion increased significantly over time (F5,13.38 = 11.652, p < 0.001), but post hoc pair-wise comparisons did not show a significant increase for any specific time point. Conscientiousness increased over time with uncorrected significance (F5,18.72 = 3.867; p = 0.014). We found no significant change over time for openness (F5,18.19 = 0.964; p = 0.47) or agreeableness (F5,17.96 = 1.399; p = 0.27). Changes in participants’ personality-dimension t scores over time compared to normative data are given in Figure 2B. Post hoc pair-wise comparisons are provided in Appendix 1, Table S2. Add-on cognitive behavioural therapy did not change the analysis of personality scores (Appendix 1, Table S4).
Personality changes over time. (A) Changes in raw personality scores from baseline to 15 months after deep brain stimulation. Baseline scores denote the significance of omnibus test results, and scores at specific time points denote the significance of post hoc test results. ***p < 0.001; **p < 0.01. (B) Changes in personality t scores; grey shaded area represents the range of the normative data.
Relationship between depression and personality scores
Depression severity (HDRS) was positively correlated with neuroticism scores at baseline (r = 0.50, p = 0.02), but this correlation was not significant after Bonferroni correction for 5 personality dimensions. The other 4 personality dimensions were not correlated with HDRS scores at baseline: extraversion (r = −0.18, p = 0.45), openness (r = −0.26 p = 0.25), agreeableness (r = −0.08, p = 0.73) and conscientiousness (r = 0.19, p = 0.40).
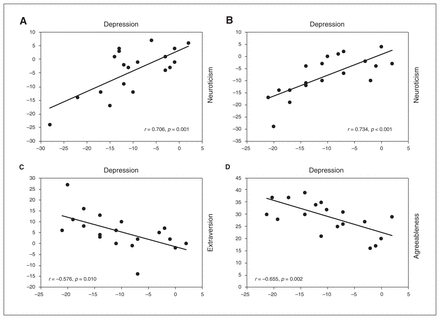
At 6 months, the decrease in HDRS score from baseline was significantly correlated with a decrease in neuroticism from baseline (r = 0.706, p = 0.001; Figure 3A). We found no significant correlation after Bonferroni correction between a decrease in HDRS scores and an increase in extraversion (r = −0.475, p = 0.03) or conscientiousness (r = −0.526, p = 0.017). However, although these correlations were nonsignificant, their strength or effect size was in the medium range (r = 0.4 to 0.5) for extraversion and large (r = 0.5 to 1) for conscientiousness.
Correlation between depression scores and personality dimensions. (A) Correlation analysis for change in depression (Hamilton Depression Rating Scale) and neuroticism from 0 to 6 months. (B) Correlation analysis for change in depression and neuroticism from 0 to 12 months. (C) Correlation analysis for change in depression and extraversion from 0 to 12 months. (D) Correlation analysis for change in agreeableness from 0 to 12 months.
We found no significant correlation at 6 months between change in HDRS scores and openness (r = −0.259, p = 0.27) or agreeableness (r = −0.193, p = 0.43).
At 12 months, decreases in HDRS score were significantly correlated with decreases in neuroticism (r = 0.734, p < 0.001; Figure 3B) and increases in extraversion (r = −0.576, p = 0.01; Figure 3C) after Bonferroni correction. We found no significant correlation between decreases in HDRS scores and increases in conscientiousness (r = −0.456, p = 0.05), openness (r = −0.393, p = 0.10) or agreeableness (r = −0.209, p = 0.41). Although these correlations were nonsignificant after correction, the effect size of the correlation between conscientiousness and HDRS scores was in the medium range (r = 0.4 to 0.5). Furthermore, linear mixed model analysis showed a significant effect of the time × HDRS interaction on neuroticism (F6,48 = 21.19, p < 0.001) and extraversion (F6,14 = 7.84, p = 0.001), suggesting that the decrease in neuroticism and increase in extraversion over time were influenced by HDRS scores.
Personality predictors of response to SCC-DBS
Response to DBS at 6 months was predicted by higher baseline openness (t18 = 3.109, p = 0.006), and response to DBS at 12 months was predicted by higher baseline agreeableness (t18 = 3.258, p = 0.004; Table 2). Baseline openness scores were not significantly correlated with reductions in HDRS scores from baseline to 6 months (r = −0.428, p = 0.06). However, baseline agreeableness was significantly correlated with a reduction in HDRS scores from baseline to 12 months (r = −0.655, p = 0.002; Figure 3D). Although age was a confounding variable, it was not correlated with either baseline agreeableness (r = −0.28; p = 0.23) or openness (r = −0.40; p = −0.08), so we did not consider it as a covariate in the prediction of clinical response to DBS.
Baseline personality predictors of response to deep brain stimulation
Subjective description of personality changes
Among 16 patients who participated in the mini structured interview, 10 patients (68%) affirmed positive personality change (Appendix 1, Figure S1, Table S3). None of the patients reported experiencing any negative personality changes. Of the 10 who identified having positive personality changes, 8 (80%) were responders.
Discussion
To our knowledge, this was the first study to prospectively analyze the effect of SCC-DBS on personality dimensions in patients with treatment-resistant depression using the NEO-FFI, a reliable and valid instrument. Our results showed that SCC-DBS was associated with positive changes in personality dimensions that were affected by depression. More importantly, these changes were related to clinical improvement, confirming the corollary nature of response and supporting state-dependent effects on these personality dimensions in depression. Furthermore, the NEO-FFI instrument may have clinical utility in the selection of patients, because higher agreeableness and openness scores predicted clinical response to SCC-DBS in treatment-resistant depression.
Consistent with our hypothesis, SCC-DBS was associated with a decrease in neuroticism and an increase in extraversion, and these changes were related to improvements in depression, suggesting state-related effects. Previous work has documented similar depression state-related changes in patients with major depressive disorder after pharmacological and somatic antidepressant treatment.15,16,18,34 Furthermore, in support of state-related effects, higher levels of neuroticism and lower levels of extraversion at baseline (relative to normative data) improved toward normative levels during the course of DBS (Figure 2). The associations of increases in neuroticism and decreases in extraversion with depressive episodes have been reported in previous studies.35,36
The concept of a state effect on personality changes suggests that personality changes observed during SCC-DBS treatment are conditional on improvement in depression and there is no direct effect of DBS on personality changes. Because the personality measure has state-effect components,37 the effect of severe depression on personality state-related components declines over time as depression improves with SCC-DBS. Furthermore, the state effect could also be explained based on an of overlap in neural mechanisms between neuroticism or extraversion and depression pathology. The overactivation of the subgenual cingulate region and amygdala in response to negative stimuli (negative bias) and diminished serotonin neurotransmission have been implicated in both neuroticism and depression.19,21,38–40 Similarly, blunted reward-processing may be a common neural mechanism for low extraversion and depression,41,42 and overactivation of the subgenual cingulate region overactivation may have a causal role in impaired reward-processing.31 However, based on our results, it was not possible to rule out the role of stimulation of the SCC in personality changes. Although the effects of SCC-DBS stimulation and personality changes were not directly related, they may not have been totally independent (i.e., nonzero correlation). It may be possible to study differences in personality changes using sham-controlled trials by examining the effect of stimulation (sham/active stimulation), treatment response (responders/nonresponders) and their interactions on personality changes. Because our study did not include a sham-control arm, we could not exclude the contribution of stimulation effects on personality changes.
We observed significantly lower levels of conscientiousness in our cohort compared to the normative data, but the increases in conscientiousness over time during SCC-DBS were not significant when corrected, probably because of the small sample size. Similarly, although the correlation was nonsignificant after correction, the strength of the relationship between increases in conscientiousness and decreases in depression severity was strong over time, with a medium to large effect size, likely also because of a lack of statistical power. Given that conscientiousness is linked to behaviours such as delayed gratification, self-discipline and cognitive control, and that the dorsolateral prefrontal cortex is a key region for the cognitive control of emotions, it is not surprising that improvement in conscientiousness was related to direct stimulation of the dorsolateral prefrontal cortex in repetitive transcranial magnetic stimulation,43 as well as indirectly through the activation of medial frontal projections in SCC-DBS.44 Contrary to a previous report,45 cognitive behavioural therapy did not affect any personality dimensions, possibly for the same reasons as its lack of effect on depressive symptoms in this cohort.6
Our study did not include healthy controls without depression or patients with treatment-responsive depression to examine the personality profile of patients with treatment-resistant depression and subsequent changes in personality with DBS relative to control groups. Nonetheless, comparison with normative data showed increases in neuroticism and decreases in extraversion and conscientiousness that exceeded or were closer to 1 SD from the normative mean, suggesting clinically meaningful change in the profile of personality in treatment-resistant depression. This finding was in line with the findings of studies involving patients with major depressive disorder.17,46 However, some studies involving patients with treatment-resistant depression showed reduced openness and extraversion as important personality deviations from normality.10,47 This suggests heterogeneity in the personality characteristics of patients with treatment-resistant depression and possible differences in selection criteria related to comorbid personality disorders and the severity of treatment-resistant depression among studies.
In contrast to the present report, a study of slMFB-DBS did not find changes in personality dimensions from baseline to 6 months, 2 years or 5 years.10 Increased scores in neuroticism and decreased scores in extraversion, conscientiousness and openness at baseline (relative to the normative sample) remained unchanged over time with slMFB-DBS treatment. This finding was interpreted as a scar effect relating to cumulative, long-term, irreversible changes associated with recurrent depressive episodes. Inconsistencies in findings between these 2 studies probably relate to differences in patient population, brain target and the timeline of assessments. The duration of the current depressive episode in the previous study was much longer (mean ± SD, 8.57 ± 7.75 years) than in our cohort (mean ± SD, 24 ± 16.5 months), which could partly explain the association of a scar effect with severe chronic depression. As well, there were differences in personality deviations from normative data between these 2 patient populations. Furthermore, the antidepressant effects of slMFB-DBS are mediated predominantly through the reward system and dopamine neurotransmission,29 and the same neurobiological systems have been implicated in extraversion.27 As such, the observed lack of changes in extraversion with slMFB-DBS was unexpected. Because the slMFB engages the reward system through dopamine neurotransmission and the SCC is a key region in regulating negative emotions through the serotonergic system,48 more studies are needed to determine the differential impact of these 2 DBS target regions on personality in treatment-resistant depression.
The qualitative assessment of patients’ perceptions of personality change in combination with quantitative measures (mixed-method approach) has been recommended to study complex constructs such as personality change.8 In our study, most of the patients (62.5%) described self-perceived positive personality changes (Appendix 1, Figure S1). Moreover, 80% were DBS responders, and they described their thoughts and behaviours as reflecting more optimism and hope in life generally, as well as improved self-confidence and self-esteem. However, these findings cannot be untangled from the amelioration of depressive symptoms such as improved energy, interest and motivation. The absence of persistent negative changes in personality is noteworthy, because it will be helpful for addressing ethical concerns and facilitating treatment decisions. The observed self-perceived positive changes in personality corresponding to clinical improvement have also been reported in slMFB-DBS for treatment-resistant depression and in DBS of the anterior limb of the internal capsule for obsessive–compulsive disorder,10,12 suggesting that self-perceived positive changes in personality might be a part of recovery.
Our results suggest that baseline agreeableness and openness scores may be associated with clinical response to DBS. Consistent with our results, agreeableness has been reported to be a predictor of clinical remission to deep transcranial magnetic stimulation for treatment-resistant depression.43 Although our findings on prediction are encouraging, the DBS study by Bewernick and colleagues10 targeting the slMFB found no evidence of personality dimensions as predictors of DBS response in treatment-resistant depression. Further evaluation and prospective validations are needed to determine the role of personality in the prediction of DBS outcomes in treatment-resistant depression. Agreeableness is a pro-social trait related to altruism, empathy and theory of mind, and it is anatomically linked to the posterior cingulate cortex and the superior temporal sulcus and temporoparietal junction.49,50 The SCC target has extensive connections with the posterior cingulate cortex through the cingulum bundle, and stimulation of cingulum fibre tracts has been associated with response to SCC-DBS.51 High agreeableness might also be considered a resource for the reestablishment of healthy supportive social relationships. The finding of openness as a predictor of DBS response was unexpected given the lack of evidence for its strong association with depression.46 However, openness to inner feelings and actions may be crucial for readiness to change and adaptability,52 and therefore could be relevant to the clinical outcomes of DBS.
Limitations
Our study had several limitations. The small sample size limits statistical inferences and generalization of our findings. Replication in large, independent samples is required to validate our findings. Nonetheless, our results were consistent with previous reports on mood state-dependent personality changes with antidepressant treatment.17,34 The lack of sham controls complicated our interpretation of whether the personality changes were related to recovery from depression regardless of active stimulation, or if these changes were related to stimulation-specific recovery. We also did not include observer or third-person (clinician and family) ratings. In the absence of observer ratings, the subjective ratings of patients may not be accurate because of possible depression-induced rating bias. Depression-related cognitive distortions can lead to overrating of neuroticism and underrating of extraversion,53 which could change with recovery from depression. Therefore, personality assessments in depression may not accurately reflect trait characteristics but mood–state characteristics instead, as artifacts of depressed mood. However, a previous slMFB-DBS study did not show state-related changes in personality, suggesting that dissociation can occur between the DBS effects on depression and personality.10 Therefore, the observed state effect on personality in the present study cannot be completely explained by rating bias. More importantly, the state-trait influences of personality in depression may be a function of comorbid personality disorder. It is known that mood-independent personality traits are more common in major depressive disorder with a personality disorder than in major depressive disorder without a personality disorder.54 Therefore, our results showing state-related personality changes may also be related to our selection of patients with treatment-resistant depression without severe comorbid personality disorders. Finally, since our findings are based on a single personality instrument, future studies should include comprehensive assessment of several aspects of personality relevant to treatment-resistant depression and DBS treatment.13
Conclusion
We found positive personality changes related to the antidepressant effects of SCC-DBS, in agreement with the mood-related effects of antidepressant treatments on personality in patients with major depressive disorder. This information may be helpful for preoperative informed decision-making. Additionally, lack of adverse personality effects in SCC-DBS reduces some of the ethical questions associated with this form of psychosurgery. Considering the predictive potential of personality assessment, more individualized screening in the selection of patients may optimize DBS outcomes. Personality assessment may have a role in clinical decision-making as well as in prognostic evaluation in future DBS trials for treatment-resistant depression.
Footnotes
Funding: Funding for the study was provided by a grant from Alberta Innovates Health Solutions, Alberta, Canada. The funding agency had no role in the study design, collection, analysis and interpretation of data; and preparation, review or approval of the manuscript. Partial data were presented as a poster at the 74th Society of Biological Psychiatry annual meeting on May 2019.
Competing interests: R. Ramasubbu has received honoraria for serving on advisory committees for Astra Zeneca, Lundbeck, Janssen and Otsuka. He also received investigator-initiated grants from Astra Zeneca and Pfizer. No other competing interests declared. Z. Kiss has received grants from the Natural Sciences and Engineering Research Council of Canada and the Dystonia Medical Research Foundation of Canada Foundation for Innovation, as well as honoraria from Elsevier, all unrelated to the present work. No other competing interests declared.
Contributors: R. Ramasubbu and Z. Kiss designed the study. R. Ramasubbu and L. McAusland acquired the data, which R. Ramasubbu, L. McAusland, S. Chopra, D. Clark and B. Bewernick analyzed. R. Ramasubbu wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received February 11, 2021.
- Revision received May 11, 2021.
- Accepted May 27, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/