Abstract
Background: The medial prefrontal cortex (mPFC) plays an important role in depression and addiction. Previous studies have shown alterations in glutamatergic activity in the mPFC following the administration of ketamine in patients with depression and healthy controls. However, it remains unclear whether chronic, nonmedical use of ketamine affects metabolites in the mPFC.
Methods: Using proton magnetic resonance spectroscopy, we measured metabolites (glutamate and glutamine [Glx]; phosphocreatine and creatine [PCr+Cr]; myo-inositol; N-acetyl-aspartate; and glycerophosphocholine and phosphocholine [GPC+PC]) in the mPFC of chronic ketamine users (n = 20) and healthy controls (n = 43). Among ketamine users, 60% consumed ketamine once per day or more, 10% consumed it every 2 days and 30% consumed it every 3 or more days. Using analysis of covariance, we evaluated between-group differences in the ratios of Glx:PCr+Cr, myo-inositol:PCr+Cr, N-acetyl-aspartate:PCr+Cr and GPC+PC:PCr+Cr.
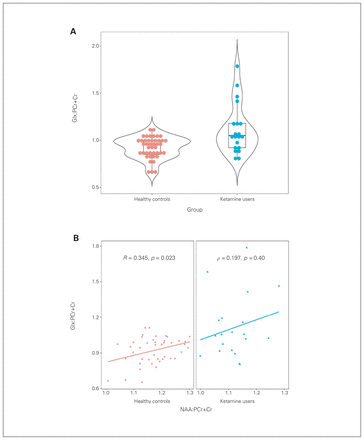
Results: Chronic ketamine users showed significantly higher Glx:PCr+Cr ratios than healthy controls (median 1.05 v. 0.95, p = 0.008). We found no significant differences in myoinositol:PCr+Cr, N-acetyl-aspartate:PCr+Cr or GPC+PC:PCr+Cr ratios between the 2 groups. We found a positive relationship between N-acetyl-aspartate:PCr+Cr and Glx:PCr+Cr ratios in the healthy control group (R = 0.345, p = 0.023), but the ketamine use group failed to show such an association (ρ = 0.197, p = 0.40).
Limitations: The cross-sectional design of this study did not permit causal inferences related to higher Glx:PCr+Cr ratios and chronic ketamine use.
Conclusion: This study provides the first evidence that chronic ketamine users have higher glutamatergic activity in the mPFC than healthy controls; this finding may provide new insights relevant to the treatment of depression with ketamine.
Introduction
Ketamine is a noncompetitive antagonist at the N-methyl-d-aspartate (NMDA) glutamate receptor and a partial agonist at μ-opioid receptors.1,2 It is widely used in human and veterinary medicine as an approved anesthetic.3 It has also shown efficacy in treating depression and suicidal thoughts in numerous studies.4,5 However, despite its important therapeutic value, ketamine also can be misused as a “club drug” that induces dissociative states and hallucinations. The recreational use of ketamine around the world has increased rapidly in the past few decades, especially among young people who attend electronic dance music parties.6 Chronic ketamine use can have physical effects, causing urological, gastrointestinal and other symptoms.7,8 It can also induce neuropsychiatric symptoms, including psychosis, depression, cognitive impairments and dependence.9,10 Brain imaging studies have revealed structural, functional and metabolite alterations in chronic ketamine users.11–13 Although no clear mechanism of chronic ketamine use has been identified, some findings have been localized to the medial prefrontal cortex (mPFC).
The mPFC is an important brain region, with afferent and efferent connections to numerous cortical and subcortical areas.14 It is involved in generating emotional responses, appraising and expressing negative emotions, decision-making and motivation through its interactions with other brain regions.15–17 The mPFC is also innervated by the glutamatergic and dopaminergic neurotransmitter systems, both of which have been implicated in addiction.18 Substantial research has documented increased glutamatergic activity in the mPFC in patients with depression, healthy participants and animal models following acute or short-term ketamine administration.19–21 However, the evidence remains sparse for whether chronic nonmedical ketamine use induces changes in the glutamatergic system in the human brain.
Glutamate is a key metabolite in synaptic activity and neuroplasticity, and glutamatergic dysfunction is thought to mediate the pathophysiology of both major depressive disorder22 and addiction.23 Normal glutamate homeostasis is modulated by a glutamate–glutamine cycle involving neurons and astrocytes.22 Presynaptic neurons release glutamate, which is taken up by astrocytes and converted to glutamine by glutamine synthetase. Neurons then take up the glutamine released from astrocytes and convert it back to glutamate via the enzyme glutaminase. Disruption of glutamate homeostasis is believed to impair the ability of people with drug addictions to control drug-seeking behaviours and regulate corticostriatal circuitry.23
Because glutamate and glutamine are closely related chemically and are poorly separated in many human in vivo studies, they are often measured together as “Glx” (glutamate and glutamine). One noninvasive method of assessing in vivo metabolite concentrations in localized brain regions is via proton magnetic resonance spectroscopy (1H-MRS).24 Several previous MRS studies have reported changes in glutamate or Glx after acute ketamine administration in healthy humans or patients with major depressive disorder. Most studies found increased Glx in the mPFC or the anterior cingulate cortex,19,20,25 although 1 reported decreased Glx levels in the mPFC in patients with major depressive disorder.26 We are aware of only 1 MRS study that has investigated chronic recreational ketamine use.27 It reported no significant difference between chronic ketamine users and polydrug users in ratios of glutamate to phosphocreatine and creatine (PCr+Cr) or Glx to PCr+Cr in the anterior cingulate cortex, left thalamus or left medial temporal cortex.
The mPFC is an important region in both depression and addiction, but we know little about metabolism changes in this area induced by chronic ketamine use, especially the alteration of glutamatergic activity. The objective of the present study was to investigate the effects of chronic ketamine use on glutamatergic activity in the mPFC in long-term (more than 1 year) chronic ketamine users. We hypothesized that we would find a significant difference between long-term chronic ketamine users (i.e., patients with ketamine dependence) and healthy controls in terms of Glx:PCr+Cr ratios in the mPFC.
Methods
Participants
We recruited 25 chronic ketamine users who were hospitalized at the Kangda Voluntary Drug Rehabilitation Centre in Hunan province, China. We also recruited 53 age- and sex-matched healthy controls from the community via advertising.
To be included, chronic ketamine users had to meet the criteria for ketamine dependence from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision,28 as assessed by the Structured Clinical Interview.29 Chronic ketamine users also had to have engaged in nonmedical ketamine use for at least 1 year, be right-handed and be aged 18–45 years. Two licensed psychiatrists conducted all clinical interviews. Exclusion criteria were as follows: other substance dependence (excluding nicotine), any comorbid psychiatric disorder, any major physical disorder (e.g., metabolic or endocrine disease, heart disease, rheumatic autoimmune disease or abnormal liver function), a history of severe head trauma, a family history of psychiatric disorders, pregnancy, breast-feeding or any contraindication to MRI.
We used the Beck Depression Inventory II to assess participants’ current depression symptoms.30 We measured trait anxiety and state anxiety using the State–Trait Anxiety Inventory.31 We used the Positive and Negative Syndrome Scale (PANSS) — which yields a positive scale score, a negative scale score and a general psychopathology scale score — to evaluate psychiatric symptoms.32
All participants provided written consent to participate in the study. The Ethics Committee of the Second Xiangya Hospital, Central South University (No. S163, 2011), approved the protocol.
MRI data acquisition
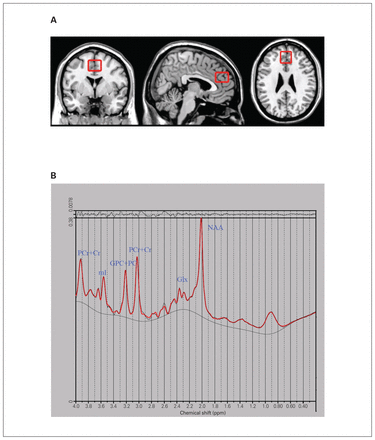
We acquired MRIs, including whole-brain structural T1-weighted MRIs (repetition time 2000 ms, echo time 2.26 ms, field of view 256 × 256 mm2, flip angle 8°, number of slices 176, slice thickness 1 mm) and localized 1H-MRS (point-resolved spectroscopy sequence, repetition time 1500 ms, echo time 30 ms, number of excitations 256, spectral bandwidth 1200 Hz, number of data points 1024), as described previously.33 All imaging was performed using a 3.0 T Siemens Trio MRI scanner and an 8-channel phased-array head coil. We placed a single MRS voxel measuring 30 × 25 × 30 mm3 in the mPFC region of interest (including the medial superior frontal and anterior cingulate cortices) dorsally adjacent to the corpus callosum (Figure 1A).33 Offline, the mPFC was segmented into grey matter, white matter and cerebrospinal fluid according to the Data Processing and Analysis of Brain Imaging protocol.34
(A) Voxel placement in the medial prefrontal cortex (30 × 25 × 30 mm3) for localized single-voxel proton magnetic resonance spectroscopy. The region of interest is shown in (left to right) sagittal, coronal and transversal orientations. (B) A representative spectrum from the medial prefrontal cortex of a healthy control participant. Glx = glutamate and glutamine; GPC+PC = glycerophosphocholine and phosphocholine; mI = myo-inositol; NAA = N-acetyl-aspartate; PCr+Cr = phosphocreatine and creatine.
We fitted the magnetic resonance spectra using LCModel version 6.3–1B.35 Indicators of data quality included signal-to-noise ratio, full width at half maximum (FWHM; spectral width at half the maximum amplitude of the PCr+Cr signal) and a Cramer–Rao lower bound value for each metabolite signal in each spectrum. Quality criteria for including data in the analysis were as follows: FWHM 0.1 ppm or less, signal-to-noise ratio 20 or greater, and Cramer–Rao lower bound value 20% or less.36,37 Because PCr+Cr is relatively stable across conditions, we used it as an internal reference. We quantified Glx, myo-inositol, N-acetyl-aspartate, and glycerophosphocholine and phosphocholine (GPC+PC) in relation to PCr+Cr using LCModel (Figure 1B). We excluded 5 ketamine users and 10 healthy controls from the final analysis because of poor data quality.
Statistical analysis
We performed statistical analysis using R 3.6.1 in RStudio 1.2.5001.38 We applied a Shapiro–Wilk test to explore the assumption of normality for each variable. We expressed group variable values as mean ± standard deviation, median (interquartile range) or frequency (percentage). We performed independent t tests, Mann–Whitney U tests and χ2 tests to compare clinical and demographic data between groups. These tests were 2-tailed, with significance set at p < 0.05 (uncorrected).
We evaluated data quality and tissue composition differences using analysis of covariance, with cigarettes smoked per day as a covariate; significance was set at p < 0.005 (0.05/10, Bonferroni correction). We also performed analysis of covariance to measure between-group differences for each metabolite (PCr+Cr, N-acetyl-aspartate:PCr+Cr ratio, GPC+PC:PCr+Cr ratio, myo-inositol:PCr+Cr ratio and Glx:PCr+Cr ratio), using cigarettes smoked per day as a covariate. Because we evaluated differences for 5 metabolites, significance was set as p < 0.01 (0.05/5, Bonferroni correction).
We performed Pearson or Spearman correlation analysis (uncorrected) to explore possible correlations between metabolite ratios and several clinical parameters: ketamine use, cigarettes smoked per day, Beck Depression Inventory II score, state anxiety score, trait anxiety score, PANSS total score, PANSS positive scale score, PANSS negative scale score and PANSS general psychopathology scale score. Because previous studies have reported a positive correlation between N-acetylaspartate and glutamate in healthy controls,39,40 we also explored the correlation between N-acetyl-aspartate:PCr+Cr and Glx:PCr+Cr ratios in both groups. We tested these analyses with an α level of 0.05.
Results
Demographic and clinical characteristics
The final analysis included 20 chronic ketamine users and 43 healthy controls. We found no significant between-group differences in terms of age, sex, years of education or body mass index (Table 1). Chronic ketamine users smoked more cigarettes per day than healthy controls (median 20 v. 0, p = 0.001). We found no significant difference in rates of alcohol use between groups (34.9% v. 55.0%, p = 0.25).
Participant demographic and clinical characteristics*
Ketamine users had a higher score on the Beck Depression Inventory II than healthy controls (median 9 v. 3, p < 0.001). We found no significant between-group differences for state anxiety total score (35.26 ± 10.38 v. 33.69 ± 9.37, p = 0.64) or trait anxiety total score (41.67 ± 10.85 v. 37.81 ± 8.12, p = 0.14).
With respect to frequency of ketamine use within the previous 30 days, 60% had used once per day or more, 10% had used every 2 days and 30% had used every 3 or more days.
MRS data quality
We found no significant between-group differences in FWHM (median 0.05 v. 0.06, p = 0.013) or signal-to-noise ratio (median 42 v. 41, p = 0.046). We also found no significant between-group differences in Cramer–Rao lower bound values for the 5 metabolites (Table 2).
MRS data quality*
Tissue composition and metabolite levels in the mPFC
We found no significant between-group differences in the volume percentage of grey matter (median 49% v. 48%, p = 0.35), white matter (median 34% v. 33%, p = 0.48) or cerebrospinal fluid (median 16% v. 17%, p = 0.44) in the mPFC (Table 2).
We found no significant between-group differences in PCr+Cr levels (6.34 ± 0.59 IU v. 6.32 ± 0.63 IU, p = 0.97). Chronic ketamine users showed significantly higher Glx:PCr+Cr ratios (median 1.05 v. 0.95, p = 0.008) in the mPFC compared to healthy controls (Figure 2A), but we found no significant between-group differences in N-acetyl-aspartate:PCr+Cr (1.12 ± 0.07 v. 1.17 ± 0.07, p = 0.06), GPC+PC:PCr+Cr (0.24 ± 0.02 v. 0.27 ± 0.03, p = 0.01) or myo-inositol:PCr+Cr (0.75 ± 0.08 v. 0.81 ± 0.08, p = 0.17) ratios (Table 3).
(A) Dot plot for Glx:PCr+Cr for both groups. (B) Glx:PCr+Cr was positively associated with NAA:PCr+Cr in healthy controls; the ketamine group failed to show this association. Glx = glutamate and glutamine; NAA = N-acetyl-aspartate; PCr+Cr = phosphocreatine and creatine.
Metabolites in the medial prefrontal cortex*
Correlation analysis
Within the chronic ketamine use group, we found no significant correlation between metabolite ratios and ketamine use, cigarettes smoked per day, Beck Depression Inventory II score, state anxiety score, trait anxiety score, PANSS total score, PANSS positive scale score, PANSS negative scale score or PANSS general psychopathology scale score.
We did find a positive relationship between N-acetylaspartate:PCr+Cr ratio and Glx:PCr+Cr ratio in healthy controls (R = 0.345, p = 0.023; Figure 2B). Ketamine users did not show such an association (ρ = 0.197, p = 0.40).
For additional analyses, see Appendix 1, available at www.jpn.ca/lookup/doi/10.1503/jpn.210179/tab-related-content.
Discussion
Our study revealed that chronic ketamine users had higher Glx:PCr+Cr ratios in the mPFC than healthy controls. Furthermore, we found a positive correlation between Glx:PCr+Cr and N-acetyl-aspartate:PCr+Cr ratios in healthy controls but not in chronic ketamine users.
The median Glx:PCr+Cr ratio in the mPFC was 10.5% higher in chronic ketamine users than in healthy controls. Previous MRS studies of the effects of acute ketamine administration on regional glutamatergic neurometabolites have had variable results, some showing an increase in Glx or glutamate19,41 but others reporting no significant changes.42,43 Disparities between studies may have been because of differences in sample size or brain region interrogated.
The single previous MRS study in chronic ketamine users found no significant difference in Glx:total creatine or glutamate:total creatine ratios compared to polydrug users.27 Our results were consistent with the idea of a role for the glutamatergic system in chronic ketamine use. Several possible mechanisms may contribute to elevated Glx in the ketamine user group in the present study. First, ketamine blocks NMDA receptors on GABAergic interneurons, which decreases spontaneous inhibitory postsynaptic currents and increases spontaneous excitatory postsynaptic currents on pyramidal neurons, resulting in increased glutamate.44 Second, ketamine blocks NMDA receptors on pyramidal neurons, inducing a compensatory increase in glutamate.45 Third, ketamine activates nicotinamide adenine dinucleotide phosphate oxidase, which undermines the ability to control glutamate release.46 Fourth, ketamine inhibits plasma membrane calcium pumps and reduces glutamate reuptake by type 2 excitatory amino acid transporters, leading to durable increases in glutamate synthesis and release.47
Previous studies have shown that a single subanesthetic dose of ketamine rapidly but transiently increases glutamate levels, but the findings in the present study indicate that effects on glutamatergic neurometabolites may persist with long-term or high-dose ketamine use (nonmedical ketamine users often used doses 5 times higher than that for depression treatment). Although median Beck Depression Inventory II scores in both groups were within the normal range in the present study, scores were higher in chronic ketamine users. We also found no significant association between Beck Depression Inventory II scores and Glx:PCr+Cr ratios in chronic ketamine users. Thus, long-term elevation of glutamatergic compounds in the mPFC with chronic ketamine use may be independent of the antidepressant effects of ketamine in the acute clinical setting.
Excessive Glx may lead to excitotoxicity, which has been related to psychotic symptoms20,27 and cognitive deficits.48 Ketamine-induced psychotic symptoms may share a neurochemical with the antidepressant effects of ketamine.20 Ketamine intoxication has been widely used as an animal model for schizophrenia.49 Studies in patients with schizophrenia or in people at risk for psychosis have variously found increased,50,51 decreased52 or normal53,54 levels of glutamate or Glx in the mPFC. Previous studies have revealed that acute ketamine administration is associated with psychotic symptoms in healthy participants55 and patients with major depressive disorder.56 Although we found no significant association between Glx:PCr+Cr ratios and PANSS scores in the present study, we cannot rule out an association between the Glx:PCr+Cr elevation we observed and the psychotic effects of chronic ketamine use. Future study is needed to explore the dynamic changes in psychotic symptoms, Glx:PCr+Cr ratios and their relationship in chronic ketamine users and patients undergoing ketamine treatment.
Repeated treatment with ketamine, even at a low dose, could come with risks of addiction and cognitive decline,57,58 which must be weighed against its antidepressant benefits. Previous studies have revealed that glutamate plays a crucial role in drug dependence and addiction, influencing reinforcement, sensitization, craving, reward and relapse.59,60 Glutamate, or the glutamate:PCr+Cr ratio, is higher in the mPFC of people addicted to prescription opioids and methamphetamine.33,61 Smokers also show lower levels of glutamate in the mPFC than nonsmokers.62
Research addressing the effects of chronic ketamine exposure on brain metabolism is sparse, consisting of 1 study.27 In the present study, a higher Glx:PCr+Cr ratio in the mPFC with chronic ketamine use may have been related to hypofunction of the glutamate transmission system. Long-term exposure to ketamine led to dysfunction of glutamate homeostasis in the mPFC of rats, with reduced expression of NMDA receptors and metabotropic glutamate receptor 5,63 resembling findings in schizophrenia.64,65 Furthermore, a reduction in rodent glutamate receptor subunits after chronic ketamine administration was still observed 4 weeks after the last injection.66 The long-term elevation of Glx in chronic ketamine users in the present study, and in other people with substance use disorders, might be associated with an impaired ability to regulate drug-seeking behaviour. In contrast, previous studies in mice and rats reported that the antidepressant effects of ketamine did not persist.67,68 Because there is overlap between the neurotransmitter system and the molecular mechanisms involved in the addictive and antidepressant effects of ketamine,69 future studies should focus on characterizing these substrates more precisely.
The positive association between Glx:PCr+Cr and N-acetylaspartate:PCr+Cr ratios observed in healthy controls in the present study was consistent with previous studies.39,40 Glutamate and N-acetyl-aspartate levels are tightly connected in neurophysiology; the 2 metabolites interconvert via the tricarboxylic acid and glutamate–glutamine cycles.70,71 The failure to observe a positive association between Glx:PCr+Cr and N-acetyl-aspartate:PCr+Cr ratios in ketamine users in the present study may be an indirect sign of underlying pathology. Previous studies have reported in vitro neuron mitochondrial dys-function and morphological changes72,73 after exposure to ketamine. Ketamine administration can also influence glutamatergic neurotransmission.74,75 A disrupted association between regional brain levels of glutamate and N-acetyl-aspartate has been reported in other diseases, such as schizophrenia and methamphetamine dependent disorder,39,40,53 suggesting that this finding is not specific to ketamine use disorder.
Limitations
This study had several limitations. The chronic ketamine users smoked more cigarettes than healthy controls. A previous study reported that smokers showed lower regional brain glutamate levels than nonsmokers.62 Therefore, we used cigarettes smoked per day as a covariate to control for this potential confounding effect. Moreover, we found no significant correlation between Glx:PCr+Cr ratio and cigarettes smoked per day. Chronic ketamine users in our study also used other substances concurrently, which may have confounded results. We did not acquire urine tests before MRI scanning, but all ketamine users underwent urine tests in the rehabilitation centre, and we checked records to confirm that other substances were not a major factor. However, to minimize confounding effects, participants were excluded for dependence on other substances.
We could not obtain a specific duration of abstinence values for most ketamine users, limiting our ability to explore potential relationships between Glx:PCr+Cr ratio and the duration of abstinence. As well, because this was a cross-sectional study, it was unclear whether the higher Glx:PCr+Cr ratio in chronic ketamine users was present before their nonmedical ketamine use or was the result of chronic ketamine use, and whether the alterations would persist if their ketamine use ended. Future longitudinal studies are needed to explore a potential causal relationship between alterations in Glx:PCr+Cr ratio and ketamine use.
Because the sex breakdown in our sample was unbalanced, we were unable to explore sex differences with confidence. When we corrected for sex in our analysis, results were similar to the findings of the present study.
It is very difficult to avoid all medication, especially medications for ketamine-associated urinary tract dysfunction. Participants used only psychotropic medications (such as benzodiazepines for sleeping) for a very short period.
We used Glx instead of glutamate in the present study because glutamate is often poorly resolved from glutamine at 3.0 T. We cannot be certain whether the present results reflect increased glutamate, increased glutamine, or both, with ketamine use. Future studies should use alternative techniques to quantify glutamate and glutamine separately.
Finally, sample sizes are unbalanced across the 2 groups, which may have introduced potential bias. We aim to recruit more chronic ketamine users to further validate the findings of the present study.
Conclusion
This study provides evidence that chronic ketamine users have elevated Glx:PCr+Cr ratios in the mPFC compared to healthy controls. One remaining question is the causal relationship between elevated Glx:PCr+Cr and chronic ketamine use. Longitudinal studies may help to elucidate this relationship.
Acknowledgement
The authors acknowledge all professionals at the Kangda Voluntary Drug Rehabilitation Centre, who helped substantially with data collection. They also thank all study participants.
Footnotes
Competing interests: None declared.
Contributors: J. Liu, T. Liu, W. Hao and Y. Liao designed the study. C. Qi and A. Xie acquired the data, which Q. Wu, J. Tang and J. O’Neill analyzed. Q. Wu, J. O’Neill and Y. Liao wrote the article, which J. Tang, C. Qi, A. Xie, J. Liu, T. Liu and W. Hao reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Funding: This study was supported by National Natural Science Foundation of China (grant no. 81671325 to Y. Liao and 81671324 to T. Liu), and the Hunan Provincial Natural Science Foundation of China (grant nos. 2020JJ4794 to Y. Liao, 2020JJ4795 to T. Liu and 2020JJ5306 to A. Xie) and Hunan Provincial Innovation Foundation for Postgraduates (grant no. CX2017B071 to Q. Wu).
- Received October 17, 2021.
- Revision received February 13, 2022.
- Revision received March 28, 2022.
- Accepted April 13, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/