Bipolar disorder (BD) is a lifelong neuropsychiatric disorder that exerts a substantial personal and public health toll, an elevated lifetime risk of suicide,1 and a remarkable degree of heterogeneity or diversity (we use these terms interchangeably). Existing studies of heterogeneity in BD tend to focus on variability or clustering along a limited number of features or domains.2 However, identifying heterogeneity across a limited number of features does not necessarily help us characterize the overall heterogeneity of BD across levels of analysis (e.g., genetic, molecular, psychological, clinical). Until recently, it was not clear how heterogeneity should best be measured or conceptualized,2,3 thereby limiting our ability to understand the diversity of BD in a holistic sense. In addition to these recent advancements in heterogeneity measurement,4,5 understanding the diversity of BD requires us to develop research strategies and methods specific to this purpose.
In this editorial, we argue that understanding the diversity of BD involves characterizing its nature, causes and consequences. The nature of diversity refers to the different ways in which BD can manifest. The causes of heterogeneity are the mechanisms by which diversity itself arises in BD. Finally, the consequences of diversity are the effects on management, diagnosis and understanding of BD attributable to the condition’s heterogeneity. We propose a framework for understanding heterogeneity in BD by inducing principles of diversity from other disciplines — chiefly statistical conservation ecology, which is concerned with the analysis of the nature, mechanisms and consequences of biodiversity.6 This framework involves the pursuit and integration of 6 methodological solutions, akin to the stages proposed by Robins and Guze,7 which we summarize using the acronym “FIELDS”: family studies, interventional studies, environmental characterization, longitudinal studies, detailed multimodal phenotyping, and statistical and computational advances. Ultimately, we hope this roadmap will help us understand the remarkable diversity of BD.
Nature of heterogeneity in bipolar disorder
One can show that the proper measure of heterogeneity is the effective number of configurations in which one can find a system.2,4,8 Here, our system of interest consists of randomly sampled individuals with BD. Now we must ask, “what constitutes a ‘configuration’?” In other words, what feature(s) must we record to identify different BD phenotypes uniquely? Ecologists may label organisms in an environment with their taxonomic classes, which implies that the species label of any given organism is the relevant configuration along which organisms vary. Ecologists would then measure the heterogeneity of an ecosystem as the effective number of species. However, ecologists often use more complex configuration identifiers, such as organisms’ functional properties and genotypes. Configuration labels can be multidimensional, and they can also be continuous, discrete, or both. What matters is that one’s configuration definition includes all relevant information necessary to quantify variation. In this section, we argue that relevant configurations of BD require measuring features across multiple levels of analysis, from genotypic and family history data to clinical presentations and environmental differences.
Clinical phenotypic heterogeneity
Bipolar disorder is defined by the evolution of a multidimensional clinical phenotype (i.e., clinical syndrome) over time. By the presence or absence of Diagnostic and Statistical Manualof Mental Disorders (DSM)9 criteria alone, using the formula presented by Nunes and colleagues,2 one can show that BD-II has 37 001 distinct phenotypic presentations. In contrast, BD-I with a single mixed episode with catatonic features can present in 148 633 017 different ways. These numbers overestimate the effective number of DSM presentations since features of mania and depression tend to co-occur, perhaps according to a lower-dimensional factor structure. They simultaneously underestimate the true diversity of BD since there are many other clinical factors relevant to the BD phenotype that are not part of the diagnostic criteria and that show variation across patients.
Familial and genetic heterogeneity
Family studies have shown substantial diversity of phenotypic and comorbidity patterns in relatives of BD probands,10,11 and early molecular genetic studies showed significant heterogeneity in the potential genetic etiology of BD.12 Modern genome-wide association studies (GWAS) have revealed effect size distributions for additive effects of common variants on the expression of BD diagnosis13 and lithium response14 in large clinical samples. Still, distinct multivariate combinations of genetic risk variants may better characterize subgroups of these cohorts.15 Novel analytical approaches, such as MiXeR, which attempt to quantify polygenic overlap beyond linear correlation,13,16 may help us to identify distinct multivariate genetic profiles within the broad class of patients diagnosed with BD. Notwithstanding, phenotypic heterogeneity makes it difficult to infer the genetic architecture of psychopathology based on GWAS data alone.17
Molecular heterogeneity
Many molecular features may be relevant to understanding both state and trait expression of BD, including signs of “accelerated aging,”18 epigenetic markers,19 and variation in second messenger systems and cellular calcium handling. These systems may shed light on many aspects of BD, from its genetic overlap with schizophrenia to the mechanisms of mood stabilizers and calcium channel antagonists, and the neurobiology of hippocampal and cognitive impairments.20–23 Importantly, second messenger signalling cascades are likely to change in a mood state–dependent fashion, which would necessitate longitudinal measurements.24 Extensive variability in the expression of microRNAs ( miRNAs) across brain regions and studies may also be a salient form of biological heterogeneity in BD.25 Given their putative involvement in synaptic plasticity, miRNAs may be of particular relevance toward understanding the translational neurobiology of BD.25
Synaptic, cellular, circuit and structural heterogeneity
Many lines of evidence suggest that changes in synaptic plasticity contribute to the pathophysiology of BD and its treatment.26 These include variations in dendritic branching, length and spine density27,28 as well as lithium-induced changes in synaptic plasticity (for example, altering the synaptic AMPA–NMDA receptor ratio).29 Dentate gyrus granule cell-like neurons cultured from induced pluripotent stem cells of patients with BD suggest hyperexcitability of glutamatergic cells in patients with BD compared with healthy controls.30 This hyperexcitability may be reversed by exposure to the mood stabilizer to which a given patient was clinically responsive.
Large-scale studies have shown that BD is associated with reductions in the volume of the hippocampus, amygdala, thalamus, left pars opercularis, left fusiform gyrus and left rostral middle frontal cortex, and increased ventricle volume compared with healthy controls, albeit with significant heterogeneity (ranging from 19.4% for amygdalar volume to 65.4% for lateral ventricular volume).31,32 Cohorts with BD also show compromised white matter integrity.33 A substantial amount of heterogeneity may be related to medication use, illness duration, number of episodes and familial patterns of genetic risk.34
Functional neuroimaging studies have consistently shown increased limbic region activity during emotional processing tasks and reduced connectivity between the amygdala and prefrontal cortical regions.35,36 However, there is considerable diversity in findings across studies, depending on mood state, experimental conditions (e.g., resting state v. task-based) and illness stage.35,36
Physiological heterogeneity
Variation in multiple physiological systems is associated with BD, including the hypothalamic–pituitary–adrenal (HPA) axis, circadian rhythms, glycemic and metabolic control systems, immune/inflammatory systems, and the gut microbiome. Corticosteroid therapy has well-known adverse effects of hypomania/mania or depression, depending on treatment duration.37 Bipolar disorder is also associated with cortisol nonsuppression on the dexamethasone suppression test and elevated diurnal cortisol secretion that may be associated with symptom profiles and lithium responsiveness.38
Circadian abnormalities are also relevant for BD, which reliably shows variations in sleep/wake cycle, activity, chronotype and circadian pacemaker gene mutations.39 Circadian abnormalities are also relevant for predicting lithium responsiveness.39
Metabolic and immunological factors are relevant for understanding the etiology and progression of BD as well as the striking correlations between BD and obesity,40 insulin resistance or diabetes mellitus,41 and cardiovascular mortality.42 Patients with BD and comorbid insulin resistance or diabetes mellitus show a higher prevalence of rapid cycling, chronic course, lithium nonresponse, functional impairment, smaller hippocampal volumes and neuronal loss.41,43,44 Immunological and inflammatory factors may be a link between BD and metabolic disorders.45
The gut microbiome may distinguish patients with BD from those with unipolar depression and healthy controls.46 Further, probands and unaffected relatives show similar gut microbiome composition that differs from nonpsychiatric controls.47 Investigations into microbiome differences in BD reveal associations with clinical dimensions. For instance, the fractional representation of the bacterium Faecalibacterium in individuals with BD negatively correlates with the severity of illness.48 Gut microbiomics in BD is a relatively new field, but preliminary results suggest probiotic treatment could improve cognitive function49 and reduce rehospitalization rates.50
Psychological and neurocognitive heterogeneity
Multiple meta-analyses have shown that BD is associated with deficits in executive functioning, verbal memory, attention, processing speed and verbal fluency,51 with some deficits found in unaffected relatives.52 Cognitive deficits may vary based on the stage of illness, clinical features (e.g., clinical course, history of psychosis, education level, intelligence, childhood adversity, metabolic disorders) and medication use.51 Clustering studies do not evince specific domains of dysfunction, but rather groups of patients with widespread cognitive abnormalities that differ mainly in severity.53 Understanding this diversity may facilitate the development of psychological and biological therapies for cognitive impairment in BD.54
Environmental heterogeneity
The development and expression of BD may be influenced significantly by environmental factors, such as light exposure, which may mediate variation in age of onset, suicidality and symptom burden/severity.39,55 Other geographically varying factors, such as cultural practices and beliefs related to mental illness, differential access to mental health services, or availability of substances, may also help explain the wide variation in global BD prevalence (ranging from 0.3% in Asia to 1.6% in South America56). Gestational infections, maternal smoking, birth complications, childhood trauma and various life events may also affect the course of illness in BD.57 In particular, childhood trauma is associated with an earlier onset, rapid cycling, lifetime suicidality, substance misuse and a larger number of lifetime mood episodes, particularly in females.57
Causes of heterogeneity in bipolar disorder
The causes of heterogeneity in BD include endogenous factors (internal to the individual) and exogenous factors (external to the individual). These are further subdivided according to the mechanistic processes by which they generate heterogeneity. Here, we subdivide heterogeneity-generating mechanisms according to a common ecological framework.58–60 Specifically, we consider allopatric, parapatric and sympatric diversity generators.
Allopatric diversity generators separate a population into discrete subgroups that progressively diverge thereafter. Endogenous allopatric diversity generators in BD may include polygenic variation with discrete effects, sex differences and comorbidities, such as traumatic brain injuries or language disorders. Exogenous iatrogenic sources may include variations in diagnostic practices and treatment exposure. Exogenous noniatrogenic sources may include major adverse life events or occupational choices, such as shift work. Discrete differences in geographic distributions may also induce diversity by limiting access to health care resources.
Parapatric diversity generators apply positive selection to the extremes of some continuously varying traits and negative selection to intermediate phenotypes, yielding 2 discretely separated groups. Endogenous parapatric diversity generators in BD may include continuous liability due to small additive genetic effects. Variation in personality structure may predispose some to substance use disorders,61 which could alter the clinical course of BD. Exogenous iatrogenic sources may involve variation in the importance assigned to specific features of the BD syndrome, resulting in diagnostic heterogeneity. An example of an exogenous non-iatrogenic source is that of solar insolation gradients.39,55
Sympatric diversity generators are more difficult to understand and detect, as they involve ostensibly spontaneous emergence of a distinct phenotype from an otherwise homogeneous baseline group. One possible endogenous sympatric diversity generator is the effect of de novo rare variants across generations. The formation and dissolution of social communities could be an important sympatric diversity generator in the modern era. The increased time spent online, coupled with stigma, social polarization dynamics and social contagion effects, may cause patients with BD to join real-world or online communities with distinct or isolated subcultures. Some groups offer positive connections and support, while others may produce, exacerbate, or reinforce maladaptive beliefs or behaviours.62
The allopatric, parapatric and sympatric diversity generation framework is imperfect.58 However, it is a useful starting point to glean 2 fundamental principles: diversity is a phenomenon that evolves by the interaction of multiple different variables, both endogenous and exogenous to the individual.
Consequences of heterogeneity in bipolar disorder
Illness progression and natural history
Individuals with diverse BD phenotypes may adapt to widely varying circumstances in their environments, which may feed back to alter their phenotype. These changing phenotypic profiles will need to adapt further to continuously changing environments, perpetuating this cycle of divergent illness progression across patients. For example, patients diagnosed with mixed features may be more likely to receive treatment with atypical antipsychotics.63 Individuals receiving such treatments may experience variable patterns of functional limitations and illness progression through insulin resistance, inflammation and structural brain abnormalities.41,43
Diagnosis, management and research
With thousands of distinct presentations that overlap with those of other syndromes as well as temporal evolution characterized by the predominance of index depressive episodes,64 the diagnostic reliability of BD is low.65
This phenotypic heterogeneity may also limit our ability to optimize early treatment of BD. While lithium response can be well predicted based on clinical features, samples of responders and nonresponders may themselves be phenotypically diverse groups, which limits the accuracy and reliability of diagnostic and treatment-related prediction models.66,67
A roadmap for understanding heterogeneity in bipolar disorder
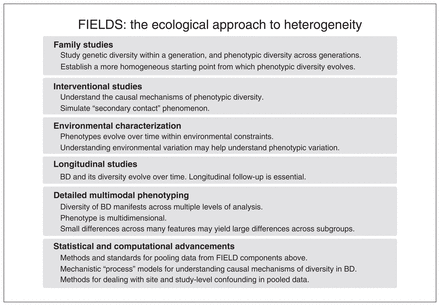
In this section, we propose a multifaceted approach to understanding the nature, causes and consequences of heterogeneity in BD. This approach involves the 6 FIELDS elements (Figure 1).
Summary of the FIELDS (family studies, interventional studies, environmental characteriszation, longitudinal studies, detailed multimodal phenotyping, and statistical and computational advancements) approach to understanding heterogeneity in bipolar disorder (BD).
Family studies
Understanding the evolution of diversity requires establishing a more homogeneous starting point from which to begin measurements. Since genetic variation is a major contributor to allopatric and parapatric diversity generators, we must seek to design research that controls for this factor. Although large-scale molecular genetic approaches have made important contributions to our understanding of the genetic architecture of psychiatric disorders, they can typically account only for the additive effects of common variants. Conversely, since relatives share larger aspects of their genetic architecture, including nonadditive genetic effects, family studies allow us to begin with more homogeneous initial conditions from which phenotypic diversity may evolve. To this end, it will be important to conduct family studies with relatives both concordant and discordant for various illness characteristics and to measure such variation over time.
Interventional studies
Interventional studies are essential to understand the causal mechanisms underlying any natural system. Specifically, concerning understanding the diversity of BD, interventional studies may help us simulate an analogous situation to the ecological phenomenon of “secondary contact,” whereby the reunion of 2 groups with a common ancestor tests whether species divergence has occurred. Analogously, we may be interested in the degree to which phenotypic variation in BD is stable/permanent or whether the ongoing presence of modifiable factors maintains this diversity.
For example, one may combine longitudinal and interventional protocols to study the evolution of phenotypic diversity in a sample of patients with BD who were phenotypically or genetically similar at the outset of their disease, showed phenotypic divergence over time, and are subsequently exposed to a randomized treatment trial. Suppose phenotypic diversity pretreatment is the product of ongoing modifiable disease activity without fundamental divergence in illness trajectories. In that case, phenotypic diversity should decline after successful treatment, and patients’ disease courses should subsequently evolve more homogeneously. So long as the outcome defining the success of the homogenizing intervention is nontrivial (e.g., a sustained period of euthymia with concordant phenotypic profiles), such trials may also help us understand the diversity of BD by comparing the degree to which medication responsiveness diverges over time within and across individuals.
Environmental characterization
Since variations in exogenous factors can cause heterogeneity, studies must characterize these features over time alongside phenotypic traits. Notable examples include studies on the effects of sunlight exposure on phenotypic expression of BD.39,55 Merging environmental characterization with family studies could involve the ongoing use of twin and adoption paradigms, specifically examining phenotypic variation between twins concordant for BD reared together compared with twins reared apart. Accomplishing such studies with sufficient power would require large-scale global collaborations.
Longitudinal studies
Since the diversity of BD evolves, we need longitudinal studies to appreciate its nature, causes and consequences. Specifically, studies of BD must cover all relevant life and illness stages, including early development, premorbid and prodromal phases through to the first episode, illness progression and end of life. Longitudinal studies must also cover depressed, manic/hypomanic and euthymic phases of the illness.
Detailed multimodal phenotyping
The section on the nature of heterogeneity in BD provided a broad, though incomplete, overview of features associated with BD and its diversity across multiple levels of analysis. It is likely that different combinations of these features underlie the expression of BD across different subgroups, or form a continuum across the BD population. To better understand this, we must embrace protocols involving collection of multimodal data that facilitate discovery of causal mechanisms spanning levels of analysis. An example of such a project is the Pharmacogenomics of Bipolar Disorder study.68 Simultaneously, we must bring together clinical and basic scientists, as well as computational modelling groups to investigate the causal mechanisms underlying covariation between features at lower levels of analysis. For instance, neuroimaging researchers have begun evaluating drivers of brain structural diversity in psychiatric disorders, finding important links to metabolic abnormalities.69 Several groups have also proposed computational models to explain mechanistic variation in mood and activity levels over time in BD.70 Such studies, if sufficiently constrained by high-quality data, will make important contributions toward understanding the causal mechanisms underlying the diversity of BD.
Statistical and computational advancements
To understand the nature, causes and consequences of heterogeneity using longitudinal family studies with interventional components and detailed multimodal phenotyping (including environmental characterization), we must develop computational and statistical approaches to represent, share and analyze the resulting complex data. This will pose important technical and scientific challenges involving harmonization as well as data collection, processing and storage. We must also develop technical expertise and reliable protocols for longitudinal active and passive collection of multidimensional data using wearables, smartphones and other devices.71
Understanding the diversity of BD poses important statistical questions, themselves foreshadowed by work in ecology, wherein the physical mechanisms of biodiversity generation remain unclear.72 Ecologists have recognized that descriptive and correlational studies are insufficient to understand the mechanisms of biodiversity development and have moved toward computational process models that simulate and compare different hypothesized mechanisms of ecosystem formation and collapse.72 These approaches are analogous to theory-driven computational studies of psychopathology, which fit mechanistic computational models to behavioural or biological data in psychiatric populations.73 These models make specific and testable assumptions about how the relevant data were generated. They can thus offer mechanistic explanations of phenotypic diversity with face and construct validity.
Conclusion
By drawing inspiration from ecological biodiversity studies, we can devise approaches for studying the nature, causes and consequences of heterogeneity in BD. First, we must progressively refine the precision and depth with which we characterize the BD phenotype. The resulting phenotypic profiles will be multidimensional and dynamic, and span multiple levels of analysis. Second, we must further study the general mechanisms by which phenotypic diversity itself can be generated over time, both within and across individuals. The allopatric, parapatric and sympatric mechanism classes are one such ecological approach, but they are imperfect and should be refined to suit the study of BD. Third, we must better characterize the consequences arising from the diversity of BD. This may include challenges related to diagnosis and management and positive feedback effects, whereby phenotypic diversity generates further phenotypic diversity in a compounded fashion. Finally, we must ensure that our experimental designs and supporting technologies are suitable for capturing the mechanisms of diversity evolution in BD. We proposed the 6 simultaneously implemented FIELDS domains. Ultimately, however, research specifically focused on the nature, causes and consequences of diversity in BD is in its early stages. Thus, we must refine these suggestions through further testing and experience.
Footnotes
The views expressed in this editorial are those of the author(s) and do not necessarily reflect the position of the Canadian Medical Association or its subsidiaries, the journal’s editorial board or the Canadian College of Neuropsychopharmacology.
Competing interests: None declared.
Funding: A. Nunes is funded by grants from the Dalhousie Medical Research Foundation and Research Nova Scotia. M. Alda is funded by grants from the Canadian Institutes of Health Research, ERANet Program, Genome Canada and Brain Canada.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/