Abstract
Background: Adolescents with borderline personality disorder often have cognitive impairment, but the underlying mechanism for this is not clear. This study was aimed at assessing alterations in regional homogeneity using resting-state functional MRI (fMRI) in adolescents with borderline personality disorder, and evaluating the associations between regional homogeneity and cognitive testing scores.
Methods: We enrolled 50 adolescents with borderline personality disorder (age 12–17 years) and 21 age- and sex-matched healthy controls. We performed regional homogeneity and seed-based functional connectivity analysis for both groups. We also performed correlative analysis for regional homogeneity and cognitive testing scores.
Results: Compared with healthy controls, adolescents with borderline personality disorder had reduced regional homogeneity values in the frontal cortex (including the left inferior orbitofrontal cortex and the bilateral superior frontal cortex) as well as in the left precuneus in the default mode network. Adolescents with borderline personality disorder also had higher regional homogeneity values in several cortical regions: the right middle temporal gyrus, the right cuneus, the right precentral gyrus and the left middle occipital gyrus. Regional homogeneity values in the left middle occipital gyrus, left inferior orbitofrontal cortex and right superior frontal gyrus were associated with cognitive testing scores in adolescents with borderline personality disorder. We also found increased functional connectivity between the left middle occipital gyrus and right superior frontal gyrus in adolescents with borderline personality disorder.
Limitations: This study had a modest sample size, with a possible case selection bias for patients with more severe illness. This cohort also included patients with comorbidities or taking psychotropic medications, which may have confounded study results.
Conclusion: Alterations in regional homogeneity and functional connectivity in brain regions that involve the limbic–cortical circuit could be neural correlates for cognitive impairment in adolescents with borderline personality disorder.
Introduction
Adolescent borderline personality disorder is a mental illness with a characteristic pervasive pattern of instability in affect regulation.1 Adolescents with borderline personality disorder may present with repeated self-harm and suicidal ideation, mood instability, impulsivity and unstable relationships. 1 Inability to regulate emotions in this disorder may be related to cognitive impairment,2 because cognitive function is involved in modulating emotional reactions as a response to environmental stimuli.3,4 However, previous studies in borderline personality disorder have focused primarily on adult patients, leaving a gap in knowledge related to cognitive function and neural mechanisms in adolescents, a distinctive subgroup.
Regional homogeneity is a data-driven analysis of restingstate functional MRI (fMRI).5 Regional homogeneity indicates synchronized activation among nearby voxels, mirroring the functional integration of neural regions. Regional homogeneity values less than the mean degree of the whole brain represent reduced synchronization of regional neural activity, and regional homogeneity values greater than the mean degree represent increased synchronization.6 Alterations in regional homogeneity have been shown in several psychiatric disorders, including schizophrenia, bipolar disorder and major depressive disorder.6–8
Functional abnormalities have been identified in the brain neural circuits of adolescents with borderline personality disorder. 2,9 For instance, abnormal activity has been shown in the limbic–cortical circuit and the default mode network (DMN),3,4,10 both of which are crucial in emotion regulation10,11 and in processing of a distorted self-image.12 A resting-state fMRI study found that adults with borderline personality disorder had decreased regional homogeneity in DMN regions (including the precuneus and posterior cingulate cortex) compared with healthy controls.13
To date, only 2 fMRI studies, both task-based, have focused on adolescent borderline personality disorder. One study showed increased activity in the hippocampus and superior frontal gyrus under negative emotional stimulation — as well as decreased activity in the orbitofrontal gyrus — in adolescents with borderline personality disorder compared with healthy controls.2 The other study found increased activity in the left insula, inferior frontal gyrus and DMN in adolescents with borderline personality disorder compared with healthy controls.9 These studies support the notion that abnormal activity in the limbic–cortical circuit and DMN may be an underlying neural mechanism for adolescent borderline personality disorder. No data have been published that assess neural correlates for adolescent borderline personality disorder using resting-state fMRI.
Cognitive impairment in people with borderline personality disorder has been noted in the literature,14 presenting as deficits in executive function and working memory.15,16 However, most correlative studies of fMRI and cognitive function in people with borderline personality disorder have been task-based and focused on adults.17,18 In the present study, we recruited 2 groups of sex- and age-matched adolescents: a patient group of adolescents with borderline personality disorder and a healthy control group. We analyzed regional homogeneity from resting-state fMRIs to evaluate spontaneous neural activation. We hypothesized that we would find regional homogeneity alterations in adolescents with borderline personality disorder that would be associated with cognitive function. The present study should help to identify neural correlates and imaging biomarkers for adolescent borderline personality disorder, which in turn would assist in its diagnosis and treatment.
Methods
Participants
This study was approved by the institutional review board of Xiangya Hospital, Changsha, Hunan, People’s Republic of China (2022020227). We obtained written informed consent from the parents or legal guardians of all participants.
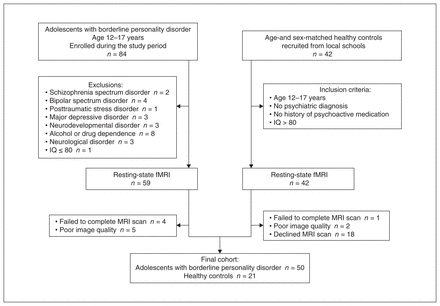
We enrolled 50 adolescents with borderline personality disorder (23 male, 27 female) and 21 age- and sex-matched healthy controls in this study. We recruited adolescents with borderline personality disorder consecutively from the psychiatric clinics at the mental health centre of Xiangya Hospital between October 2021 and February 2022. We recruited healthy controls (matched for age and sex) from local schools during the same period. Details of the patient recruitment process are presented in Figure 1.
Flow chart illustrating the enrolment process. fMRI = functional MRI; IQ = intelligence quotient.
Inclusion criteria for the patient group were as follows: patients aged 12–17 years who satisfied at least 5 of the 9 DSM-IV19 diagnostic criteria for borderline personality disorder; symptoms stable for more than 2 years; and symptoms were not better interpreted as a DSM-IV Axis I psychiatric disorder or a neurodevelopmental disorder. Patients’ scores on the Borderline Personality Feature Scale (BPFS) for children was higher than the cut-off of 66. Inclusion criteria for the healthy controls were as follows: participants aged 12–17 years; and no history of psychiatric disorders or psychotropic medication. Exclusion criteria for all participants were as follows: history of schizophrenia spectrum disorder, bipolar spectrum disorder, posttraumatic stress disorder, neurodevelopmental disorder, major depressive disorder, alcohol or drug dependence, attention-deficit/hyperactivity disorder, or neurologic disorder; or IQ of 80 or less. All participants were right-handed, based on the Edinburgh Handedness Inventory.20
Among the patient group, 80% were drug-naive (i.e., had not taken any psychotropic medication in the 2 months before enrolment) and 20% were taking psychotropic medication, including antidepressants, antipsychotics or mood stabilizers.
Structured interview and symptom assessment
All participants underwent cognitive testing and a structured clinical interview to determine whether they had a personality disorder or other psychiatric disorder. The clinical team consisted of senior psychiatrists (Q.X. and F.J.), who performed comprehensive clinical evaluations and made final diagnoses.
Patients underwent structured interviews using the borderline personality disorder section of the Structured Clinical Interview for DSM-IV Axis II Personality Disorders.19 As noted above, 5 or more of the 9 diagnostic items were required to confirm a diagnosis of borderline personality disorder. To evaluate whether patients had Axis I disorders, we used the Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime version (K-SADS-PL).21 Healthy controls underwent the same structured interviews to rule out personality disorders and Axis I disorders. Participants and their parents or legal guardians attended the structured interviews. They also provided a family history, which we used to collect information about psychiatric disorders in first-degree relatives.
We used the Abbreviated Wechsler Intelligence Scale to evaluate participants’ IQ.22 We used the Chinese version of the BPFS for children to assess the 4 core symptoms of borderline personality disorder in both groups.23 All interviews and scale evaluations were completed on the day of the restingstate fMRI brain scan.
Cognitive testing
We selected the cognitive tests used in the present study to reflect the main features of cognitive impairment in patients with borderline personality disorder, including slowed processing speed, impaired working memory and abnormal reaction inhibition. 24,25 We used the Stroop Color and Word Test (SCWT) to measure attention and response inhibition.26 It contained 3 subtasks: reading characters (SCWT-A), reading colour (SCWT-B) and reading colour disturbance (SCWT-C). We used the Trail Making Test (TMT) to measure attention and processing speed. The TMT consists of 2 subscales:27 TMT-A measures processing speed and attention, and TMT-B measures cognitive flexibility. We also used the Digit Span Test (DST): DST-A (reciting the assigned numbers forward) to measure attention and DST-B (reciting the assigned numbers backward) to assess working memory.22
Resting-state fMRI and regional homogeneity analysis
We acquired resting-state fMRI brain scans for all participants. We asked participants to avoid alcohol or psychotropic substances for 24 hours before the scan.
We obtained resting-state fMRIs using a gradient echo–echo planar imaging sequence with the following parameters: repetition time 2000 ms, thickness 4 mm, slices 30, echo time 30 ms, field of view 240 mm × 240 mm, in-plane resolution 64 × 64, gap 0.4 mm, flip angle 90°. During scanning, participants were asked to keep their eyes closed, to avoid thinking of anything and to avoid falling asleep.
We performed preprocessing of the resting-state fMRI scans using SPM12 (www.fil.ion.ucl.ac.uk/spm/software/spm12). Images with head movements greater than 3 mm or head rotation greater than 3° were excluded. We normalized the resting-state fMRI data using the SPM12 echo planar imaging model and resampled the images to 3 × 3 × 3 mm3. To decrease physiologic high-frequency noise and low-frequency drift, we used a temporal band-pass filter (0.01–0.08 Hz).
We used DPABI software (rfmri.org/DPABI) for regional homogeneity analysis. We calculated the similarity of time series using Kendall’s coefficient concordance (KCC). We calculated KCC in every participant for the time series within the special voxel and the closest 26 voxels.28 We obtained a regional homogeneity map for each participant. We used a mask from DPABI to exclude nonbrain regions and categorized the regional homogeneity maps by the overall average of the KCC values in the mask.29 Finally, to weaken noise and anatomic distinction, we smoothed the fMRI data using a Gaussian filter for 6 mm full width at half maximum.
Functional connectivity analysis
We performed a functional connectivity analysis of the restingstate fMRI data using a region-of-interest approach. We chose this approach because it was effective for investigating alterations in functional connectivity that might be correlated with cognitive function.
In the regional homogeneity analysis, we used specific brain regions as seeds to analyze functional connectivity to the whole brain. These seed regions showed altered activation in the left middle occipital gyrus and right superior frontal gyrus that have been correlated with cognitive dysfunction. We set the seeds for the functional connectivity analysis based on the results of the regional homogeneity analysis: left middle occipital gyrus (x, y, z = −18, −96, −3) and right superior frontal gyrus (x, y, z = 27, 51, 45). We then calculated Pearson correlation coefficients for each seed point and the whole brain, and performed Fisher Z transform.
Statistical analysis
We used SPSS version 22.0 (SPSS Inc.) to analyze demographic and clinical information. We used independent 2-sample t tests or Mann–Whitney U tests for continuous variables. We used χ2 tests for categorical variables.
For the regional homogeneity analysis, we performed 2-sample t tests to detect differences between the 2 participant groups. We set p < 0.05 as the statistical threshold and used family-wise error (FWE) correction (cluster size ≥ 100). The regional homogeneity analysis included sex, age and medication as covariables. Values are represented as mean ± standard deviation (SD).
We performed correlation analysis between regional homogeneity values and cognitive function using Spearman rank correlation. We controlled for age, sex, course of disease, age at onset and medication. Regional homogeneity values are represented as mean ± SD. Two-tailed statistical significance was set at p < 0.05.
The functional connectivity analysis included age and sex as covariables. Statistical thresholds were set at p < 0.001 for voxels and p < 0.05 for clusters, with FWE correction for multiple comparisons.
Results
Demographic and clinical characteristics
Participant demographic and clinical characteristics are shown in Table 1. Among the adolescents with borderline personality disorder, the median age was 14.6 ± 1.1 years (range 12–17 years), and more than half were female (n = 27; 54%). The median age at onset of borderline personality disorder was 12 years, and the median course of disease was 3 years. About half (52%) of patients had a family history of a psychiatric disorder. In the 2 months before the scan, 20% of patients reported taking psychotropic medications, including antidepressants, antipsychotic medications or mood stabilizers. A small number of patients had psychiatric comorbidities (obsessive–compulsive disorder and generalized anxiety disorder).
Demographic characteristics and cognitive testing scores*
Scores on the BPFS for children were higher in adolescents with borderline personality disorder than in healthy controls (p < 0.001). Adolescents with borderline personality disorder also had significantly more cognitive impairments than the healthy controls; we found significant between-group differences in cognitive testing scores, including the SCWT-A (p < 0.001), SCWT-B (p < 0.001), SCWT-C (p = 0.034) and DST-B (p = 0.004). We found no between-group differences in scores on the TMT-A, TMT-B or DST-A.
Regional homogeneity analysis
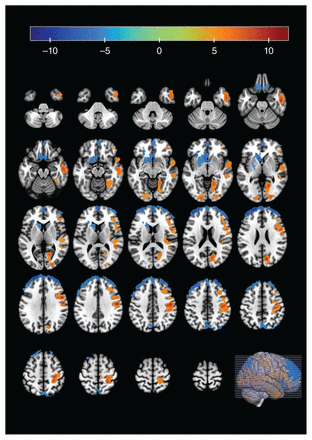
Compared with healthy controls, adolescents with borderline personality disorder showed reduced regional homogeneity in the bilateral superior frontal cortex (left: x, y, z = −21, 63, 27, t = −11.7932, cluster size = 962; right: x, y, z = 27, 51, 45, t = −10.6559, cluster size = 437), the left inferior orbitofrontal cortex (x, y, z = −24, 30, −9; t = −8.5721; cluster size = 524) and the left precuneus (x, y, z = 0, −75, 48; t = −7.244; cluster size = 218; pFWE < 0.05, cluster size ≥ 100; Table 2 and Figure 2).
Brain regions showing increased regional homogeneity (in orange) and reduced regional homogeneity (in blue) in the limbic–cortical circuit and default mode network in adolescents with borderline personality disorder compared with healthy controls.
Brain regions with altered regional homogeneity in adolescents with borderline personality disorder compared with healthy controls
Compared with healthy controls, adolescents with borderline personality disorder showed increased regional homogeneity in the right middle temporal cortex (x, y, z = 54, −12, −24; t = 9.4622; cluster size = 764), the left middle occipital cortex (x, y, z = −18, −96, −3; t = 8.4587; cluster size = 104), the right precentral cortex (x, y, z = 54, 6, 30; t = 10.5227; cluster size = 1171) and the right cuneus (x, y, z = 12, −75, 24; t = 10.0292; cluster size = 589; pFWE < 0.05, cluster size ≥ 100).
Regional homogeneity and cognitive function
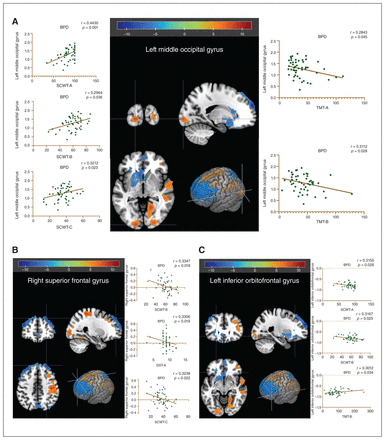
We assessed for correlations between brain regions with increased regional homogeneity values and cognitive testing scores. Regional homogeneity in the right superior frontal gyrus was correlated with attention (SCWT-B, r = −0.335, p = 0.018), response inhibition (SCWT-C, r = −0.324, p = 0.022) and digital memory (DST-A, r = −0.331, p = 0.019). Regional homogeneity in the left middle occipital gyrus was correlated with attention (SCWT-A, r = 0.443, p = 0.001; SCWT-B, r = 0.296, p = 0.036), response inhibition (SCWT-C, r = 0.321, p = 0.023), reaction speed (TMT-A, r = −0.284, p = 0.045) and cognitive flexibility (TMT-B, r = −0.311, p = 0.028). Regional homogeneity in the left inferior orbitofrontal gyrus was correlated with attention (SCWT-A, r = −0.315, p = 0.026; SCWT-B, r = −0.317, p = 0.025) and cognitive flexibility (TMT-B, r = 0.301, p = 0.034; Figure 3). In the correlation analysis we controlled for time of onset, duration of disorder, age, sex and medication load.
Correlation analysis for regional homogeneity and cognitive testing scores in adolescents with borderline personality disorder. (A) Correlation between regional homogeneity in the left middle occipital gyrus and scores on the Stroop Colour–Word Test and Trail Making Test. (B) Correlation between regional homogeneity in the right superior frontal gyrus and scores on the Stroop Colour–Word Test and Digit Span Test. (C) Correlation between regional homogeneity in the left inferior orbitofrontal gyrus and scores on the Stroop Colour–Word Test or Trail-Making Test. The threshold for significance was set at p < 0.05 (uncorrected). BPD = borderline personality disorder; DST = Digit Span Test; SCWT = Stroop Colour–Word Test; TMT = Trail-Making Test.
Functional connectivity analysis
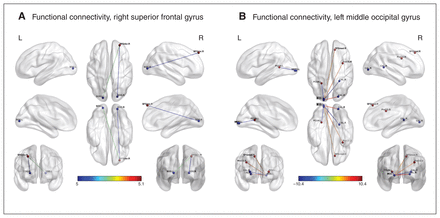
Because the left middle occipital gyrus and right superior frontal gyrus were associated with cognitive function in adolescents with borderline personality disorder in the regional homogeneity analysis, we conducted an analysis to determine whether functional connectivity was altered in these 2 regions. Compared with healthy controls, adolescents with borderline personality disorder showed increased functional connectivity between the left middle occipital gyrus and the right superior frontal gyrus (x, y, z = 12, 39, 45; t = 4.768; cluster size = 174), right precentral gyrus (x, y, z = 33, 3, 30; t = 5.7908; cluster size = 1275) and right calcarine (x, y, z = 21, −87, 0; t = 7.5804; cluster size = 239). They showed decreased functional connectivity between the left middle occipital gyrus and the left precuneus (x, y, z = −24, −51, 6; t = −8.237; cluster size = 824) and right calcarine (x, y, z = 24, −45, 6; t = −10.4017; cluster size = 219; Table 3 and Figure 4).
Significant alterations in functional connectivity in adolescents with borderline personality disorder compared with healthy controls using the right superior frontal gyrus and left middle occipital gyrus as seeds. (A) Increased functional connectivity between the right superior frontal gyrus and the bilateral superior occipital gyrus. (B) Increased functional connectivity between the left middle occipital gyrus and the right superior frontal gyrus, right precentral gyrus and right calcarine; decreased functional connectivity between the left middle occipital gyrus and the left precuneus and right calcarine. pFWE < 0.001 for voxels, pFWE < 0.05, for clusters. Colour bar indicates T scores.
Brain regions with altered functional connectivity to the seed region at the left middle occipital gyrus in adolescents with borderline personality disorder compared with healthy controls
Compared with healthy controls, adolescents with borderline personality disorder showed increased functional connectivity between the right superior frontal gyrus and the bilateral superior occipital gyrus (left: x, y, z = −18, −90, 3, t = 5.0486, cluster size = 268; right: x, y, z = 21, −87, 3, t = 5.0029, cluster size = 263; voxel p < 0.001, cluster pFWE < 0.05; Table 4 and Figure 4).
Brain regions with increased functional connectivity to the seed region in the right superior frontal gyrus in adolescents with borderline personality disorder compared with healthy controls
Discussion
In the present study, we identified altered regional homogeneity and functional connectivity in the limbic–frontal circuit and the DMN regions in adolescents with borderline personality disorder. We also found that regional homogeneity values in the frontal gyrus and middle occipital gyrus were associated with cognitive function in adolescents with borderline personality disorder. To the best of our knowledge, this was the first resting-state fMRI study to focus on adolescents with borderline personality disorder. Our results implicate regional homogeneity as a potential neural correlate underlying cognitive impairment in adolescents with borderline personality disorder.
Our finding of altered regional homogeneity in the right middle temporal gyrus was consistent with data from task-based fMRI studies in adolescents with borderline personality disorder.2,9 The middle temporal gyrus is an important part of the limbic system and is involved in emotion regulation. 30 Alterations of the middle temporal gyrus could lead to dysregulation of impulse and affect, the core symptoms of borderline personality disorder.31 We also found reduced regional homogeneity in the frontal cortex (including the bilateral superior frontal gyrus and the left orbitofrontal gyrus) in adolescents with borderline personality disorder compared with healthy controls, a finding that was generally in line with the literature. Decreased prefrontal activity has been associated a with lack of emotional and behavioural control in patients with borderline personality disorder,10 supporting the notion that a dysfunctional prefrontal cortex may lead to mood instability in patients with borderline personality disorder. 11 These results suggest that reduced synchronization of activation in the frontal cortex (as indicated by reduced regional homogeneity) may be involved in the development of borderline personality disorder in adolescents.
We also found altered regional homogeneity in the limbic–frontal circuit in our cohort of adolescents with borderline personality disorder: reduced in the bilateral superior frontal gyrus and left inferior orbitofrontal gyrus, and increased in the right middle temporal gyrus. However, similar findings have not been identified in adults with borderline personality disorder,13 indicating that these changes may be unique to adolescents and occur in the early stages of the disorder. The limbic system is involved in emotional instability, depression, impulsive behaviour and temper outbursts in patients with borderline personality disorder.11 Adolescence is the peak period for the severe symptoms of borderline personality disorder, such as psychosis, self-harm and suicidal behaviours. 1 Therefore, it was surprising to find changes in the limbic–cortical circuit in our cohort of adolescents with borderline personality disorder. We also found diverging directions in the altered regional homogeneity in our cohort: reduced in the prefrontal cortex and increased in the limbic system. A previous study has suggested that overactivation of the limbic system in patients with borderline personality disorder is because of a lack of regulation from insufficient prefrontal cortical activity.11 Our study showing diverging changes in regional homogeneity for the prefrontal cortex and the limbic system suggests a potential compensatory mechanism and neuroplasticity to maintain brain function in patients with borderline personality disorder.
To our knowledge, this study was the first to identify decreased regional homogeneity values in the precuneus in adolescents with borderline personality disorder. This finding is important, because the precuneus is a core hub of the DMN. It was consistent with previous studies on borderline personality disorder in adults,13 indicating that the precuneus may be related to psychopathology and a treatment effect of borderline personality disorder.12,32 The precuneus has been implicated in self-referential processes and is associated with the symptoms of distorted self-image and sense of abandonment in borderline personality disorder.12 Abnormal synchronization of activation in the precuneus, as reflected by alterations in regional homogeneity, could represent a dysfunctional DMN and may be a candidate imaging biomarker for borderline personality disorder.
We found that regional homogeneity values in the right superior frontal gyrus, left middle occipital gyrus and left orbitofrontal gyrus were associated with cognitive function. Previous task-based fMRI studies have observed changes in the prefrontal lobe and the orbitofrontal gyrus in adults with borderline personality disorder.17 The middle occipital gyrus had an abnormal volume in patients with borderline personality disorder,33,34 and this finding may be associated with the dissociation and hallucinations that occur in adolescents with borderline personality disorder.34,35 Abnormal activity in the middle occipital gyrus has been reported in patients with mild cognitive decline, showing correlations with scores on the Mini-Mental State Examination and Verbal Fluency Test.36 We identified brain regions associated with cognition that may be specific for adolescents with borderline personality disorder. The findings of this study support the view that borderline personality disorder in adolescents may result in more pervasive damage in the brain and lead to more severe cognitive impairment.
There are differences between the adult and adolescent forms of borderline personality disorder. Our findings showed alterations in regional homogeneity in the middle occipital gyrus in adolescents; this is less commonly reported in adults. In addition, we identified extensive cortical abnormalities in our cohort of adolescents, unlike adults with borderline personality disorder, who more often have localized abnormalities concentrated in the prefrontal lobe.33
We found a functional connection between the right superior frontal gyrus and the left middle occipital gyrus in adolescents with borderline personality disorder. Previous studies have found a decrease in the integrity of the structural connection between the prefrontal and occipital cortex, associated with cognitive impairment.37 Therefore, altered connectivity in the fronto-occipital circuit in adolescents with borderline personality disorder may be related to cognitive function. Abnormal activity in the midline subcortical nuclei, such as the ventral striatum and ventral tegmental area, has been associated with impaired emotion regulation, personality functioning and identity integration in adults with borderline personality disorder.38,39 However, we did not identify significant alterations in midline structures in our cohort of adolescents, possibly because of our modest sample size, differences in data analytical methods or differences between adults and adolescents with borderline personality disorder. Future studies should assess regional homogeneity in midline brain regions, to identify relevant neuroimaging biomarkers of cognitive function in adolescents with borderline personality disorder.
Scalabrini and colleagues40 have proposed a multilayered neuropsychodynamic model of personality organization supported by resting-state fMRI. Specifically, they indicated that impairment of the spatiotemporal structures assessed using resting-state fMRI (such as increased activity in the midline regions of the DMN and alterations in the orbitofrontal cortex) may be neuronal correlates of borderline personality disorder for emotional dysregulation and difficulty in self-referral. We also found alterations in regional homogeneity and functional connectivity in both the DMN and orbitofrontal cortex, enhancing our understanding of self and personality in adolescents with borderline personality disorder. Our findings support the proposed personality model.
Dissociation is characterized by compartmentalization and detachment symptoms, and is frequently observed in borderline personality disorder.34,35 A resting-state fMRI study of dissociation by Scalabrini and colleagues35 in a young healthy sample presented neuroimaging data that supported dissociation as a disorder of temporospatial integration of brain spontaneous activity. They showed significant negative correlations between regional homogeneity values and dissociative proneness scores, and between network functional connectivity and dissociative proneness. This study is relevant to ours because it assessed dissociation, a key symptom of borderline personality disorder. Our study also evaluated alterations in regional homogeneity and functional connectivity, although we investigated a sample of adolescents with borderline personality disorder and focused on cognitive function rather than dissociative testing. Nevertheless, our findings of altered regional homogeneity in the limbic–cortical circuit and the DMN regions (including the precuneus) were generally in line with those of Scalabrini and colleagues.35 As well, the functional connectivity changes in the middle occipital gyrus that we found in the present study were also noted in the correlation between the salience network and dissociative scores in the study by Scalabrini and colleagues.35 These converging resting-state fMRI data on regional homogeneity and functional connectivity support Scalabrini and colleagues’ proposal35 that reduced temporospatial binding and synchronization are responsible for the compartmentalization and detachment symptoms of dissociation.
Limitations
The present study had several limitations. First, we may have had case selection bias because we may have patients with more serious illness in our cohort. This was because, as a tertiary psychiatric care centre in China, our hospital routinely cares for patients referred from community hospitals. Second, our sample size was modest. We did not have sufficient statistical power to account for confounding variables related to regional homogeneity and functional connectivity. Third, we excluded people with major psychiatric illnesses such as bipolar spectrum disorders, major depressive disorder, posttraumatic stress disorder and attention-deficit/hyperactivity disorder to avoid confounding factors in data interpretation.41,42 Nevertheless, a small proportion of patients had comorbidities, including generalized anxiety disorder and obsessive–compulsive disorder. We found it challenging to exclude these comorbidities because borderline personality disorder is often accompanied by high anxiety. It was also challenging to enrol patients with no comorbid psychiatric illnesses, because the comorbidities were inseparable from the features of borderline personality disorder;43 some were even characteristics of borderline personality disorder. In our cohort, only a small percentage of patients were taking antidepressants (20%); the proportion of participants taking mood stabilizers and atypical antipsychotics was even lower. Therefore, we did not perform subgroup analyses for drug-naive and drug-treated participants; our sample size was not sufficiently powered for meaningful subgroup statistical analysis. We added medication use as a covariate to our fMRI data analysis, and similar approaches have been reported in previous MRI studies of borderline personality disorder (i.e., no additional subgroup analysis stratified by medication use).9,44,45
Conclusion
We have reported alterations in regional homogeneity and their association with cognitive function in adolescents with borderline personality disorder. Further research is needed to elucidate the specific neural mechanisms associated with these findings, and to identify robust neuroimaging biomarkers for adolescents with borderline personality disorder. Such an approach should assist in accurate diagnosis and improve prognosis for this serious mental disorder in adolescents.
Acknowledgments
We thank staff members in the department of radiology and mental health centre at Xiangya Hospital for their efforts in collecting the information used in this study, and for their assistance in manuscript preparation. We thank Dr. Zemin Tan, Department of Neurology, Xiangya Hospital, Central South University, Changsha, Hunan, P.R. China, for his guidance in neurological evaluation. We thank Dr. Daolong Zhang, Palo Alto VA Medical Center, San Francisco, Calif., USA, for his videoconference consultations to help us diagnose some of the patients with borderline personality disorder.
Footnotes
Competing interests: None declared.
Contributors: X. Yi, Z. Zhang, F. Jiang and Q. Xiao designed the study. X. Yi and Y. Fu acquired the data, which X. Yi, Y. Fu, Q. Xiao and B. Chen analyzed. X. Yi and Q. Xiao wrote the article, which all authors reviewed. All authors approved the final version to be published, agree to be accountable for all aspects of the work and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Funding: This research was funded by the Youth Fund of the National Natural Science Foundation of China (82201702), the Youth Science Foundation of Xiangya Hospital (2020Q20), the Xiangya–Peking University, Wei Ming Clinical and Rehabilitation Research Fund (No. xywm2015I35), the Natural Science Foundation of Hunan Province (2022JJ1179) and the China Post-Doctoral Science Foundation (2022M713536).
- Received August 17, 2022.
- Revision received October 4, 2022.
- Revision received October 13, 2022.
- Accepted October 14, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/