It is an error to see only illness in abnormality
— Lev Vygotsky1
Considerable variations exist in the clinical presentation of psychiatric disorders. For any given diagnostic construct, there are several thousand combinations of symptoms that can lead to the diagnosis. This considerable interindividual variation is often termed heterogeneity. Heterogeneity is not only seen at the clinical and latent (biological) levels across individuals but also apparent across time for markers such as cognition or the biophysical properties of the brain (i.e., its structure or function) in the same individual. Some of this heterogeneity is explained by how the constructs of disorders are defined in classification manuals leading to multiple combinatorial results for the same diagnosis.2 Individual components (i.e., symptoms) that form these constructs are also equally, if not more, heterogeneous.3 In other words, all psychiatric objects — diagnostic constructs, their constituent symptom units and putative biological markers — are heterogeneous in nature. Parsing this heterogeneity presents an opportunity, with calls to design stratified clinical trials to improve effect sizes4 and to abandon diagnostic constructs in favour of either latent statistical structures (e.g., searching for parsimonious features such as the p-factor, which explains variance across many disorders)5 or a complex systems perspective of psychopathology.6
Heterogeneity can be understood as deviation from a prototype;7 this may take the shape of a large range of possible observations around a prototype, or the existence of multiple prototypes (termed biotypes8 or subtypes). A recent editorial in the Journal of Psychiatry and Neuroscience dealt with the issues pertaining to the quantification of psychiatric heterogeneity.9 The current editorial addresses the interpretation and implications of an aspect of heterogeneity — brain morphometric (structural) variations — using schizophrenia as an example. Measures of brain structure based on magnetic resonance imaging (MRI) are widely used indices for subtyping, given their stability compared with symptoms, test performance scores or functional activation patterns.
Multiple mechanistic pathways
The predominant interpretation of individual differences is the presence of many mechanistic routes converging on the clinical phenotype. This suggests that each individual or subgroup may follow a distinct causal route to reach the same psychiatric characteristic. This concept of equifinality has prominently shaped heterogeneity research. It has spurred extensive endeavours in genetics (e.g., genome-wide searches for polygenic risk), brain imaging and molecular psychiatry (e.g., unsupervised clustering studies) and psychopathology (e.g., factor analytic studies). In the context of equifinality, subgroups of patients are expected to share a common causal mechanism.10 Although these subgroups may exhibit surface-level similarities, they can vary in subphenotypic attributes such as brain structure or long-term outcomes. In addition, they might respond more uniformly to treatments that target specific pathways.
Cortical impoverishment and polygenic risk of schizophrenia
Morphometric properties of various brain structures show a higher degree of between-subject variability in the presence of schizophrenia, compared with healthy controls.11,12 Clustering approaches have exploited this to show the presence of anatomic subtypes in schizophrenia, although there is no consensus on the exact number of subtypes.13–19 This is likely owing to methodological and sampling differences. Among this general lack of consensus, a striking agreement has emerged. A subgroup of patients with schizophrenia display an MRI phenotype that can be termed as cortical impoverishment, defined as reduced grey matter thickness or volume, indicating a distributed reduction in cortical tissue, particularly in the frontotemporal and parietal areas.13,15,20–22 This subgroup is apparent from very early stages to chronic established illness,20 has higher glutamate levels in prefrontal cortex,23 shows poor long-term functioning22 and has relatively higher polygenic risk scores, compared with other patient subgroups.17
In schizophrenia, converging evidence from both common and rare genetic variants of risk supports synaptic dysfunction as a key mechanism.24,25 Synaptic plasticity — the activity-dependent regulation of connectivity at the neuronal level — appears to be constitutionally defective among those at risk of this illness.26 In neurally differentiated induced pluripotent cells from patients with schizophrenia, synapses are eliminated at a higher rate, providing empirical support for the genetic predictions.27 Although the exact cellular source of the grey matter MRI signal is still unclear, there are plenty of reasons to suppose that, in schizophrenia, reduced MRI grey matter reflects reduction in dendritic spines, the seat of excitatory neuronal synapses.28 Thus, it is not surprising that high polygenic risk scores are associated with the cortical impoverishment phenotype.29
When clustering approaches are applied to pooled samples of patients and unaffected controls, it becomes evident that morphometric patterns recovered from patients are not unique to this group.14,20,22,30 The nature of variations seen among patients are the same as those seen among healthy controls, although a smaller proportion of healthy controls display cortical impoverishment.15,20 Among healthy adults, reduced brain tissue in several key areas (e.g., frontotemporal, language regions) occurs when polygenic risk scores are high.12,31 Nevertheless, the polygenic risk score does not explain the increased between-subject variability per se.12 In other words, the polygenic risk score contributes to a negative deviation from the prototypical brain structure (i.e., a 1-sided right shift in the continuous distribution of morphometric values around the norm), but this polygenic load is insufficient to account for all of the morphological variations seen in schizophrenia. As the polygenic risk score typically explains only a small amount of total disease variation, nongenetic causal factors or non-causal factors (e.g., compensatory adaptation or treatment effects) likely contribute to the observed anatomic heterogeneity.
Grey matter enrichment in schizophrenia
Some clustering studies have reported the presence of a patient subgroup with higher grey matter tissue concentrations (i.e., enrichment), mostly in the basal ganglia13,14,22,30 but also in the parieto-occipital cortex.32 A series of case–control studies have also reported an unexpected increase in brain tissue among patients and those who are predisposed to schizophrenia,33 even before any treatment exposure.34 These excesses appear as deviations from healthy norms but occur in the presence of better outcomes or less illness burden (i.e., are apparently beneficial or compensatory). A subtle but statistically significant excess of grey matter concentration occurs among patients with considerably short duration of psychotic illness,35 which is associated with less severe symptoms and better cognitive profile among untreated patients.35 Progressive supranormal deviations are also reported among adolescents with subthreshold symptoms for neurodevelopmental markers such as gyrification,36 a feature that may relate to better prognosis at later stages.37 Abnormally high volumes of grey matter are reported among at-risk individuals,33 with higher volume scaling with lower symptom burden38. This compensatory tissue excess is more apparent before illness onset, such as among those who are clinically or genetically (e.g., sibling) high risk,40,41 but is still observable (using normative approaches) among those with established illness.42 Lv and colleagues42 noted that, although polygenic risk scores were higher among patients with an overall pattern of cortical impoverishment, several regions with supra-normal thickness (> 95th percentile) were associated with polygenic risk scores. Among patients with schizophrenia, 46% had supranormal deviations of at least 1 brain region.42 Taken together, these findings indicate that a competing process, likely opposing the dominant anatomic influence of common genetic variants, is at play. Consequently, an isolated right-shift mechanism is unlikely to explain the full spectrum of anatomic heterogeneity in schizophrenia.
An interesting feature of these supranormal deviations is the distributed nature of changes, resulting in subtle overall effect sizes in case–control studies.33 Thus, these putative compensatory processes are not consistent in time, place or person. Nevertheless, concomitant structural changes of an opposing nature (i.e., a subtle increase at a distant but connected region for any localized reductions in brain tissue) are observed as a rule, not an exception, not only in schizophrenia, but across many psychiatric disorders (see a network-level synthesis by Mancuso and colleagues43). This also raises the possibility that the genetically susceptible region itself may have localized compensatory changes in an effort to escape the negative influence of the disease risk on its structure and function. In this case, both supranormal and near-normal brain structure among those with high-risk scores may be products of an interaction between causal and noncausal (adaptive) forces. These apparently disparate observations can be reconciled if the brain is considered a dynamic adaptive system, namely a set of interconnected, self-organizing elements (i.e., distributed brain networks). When such a system responds to agents that tend to destabilize it, a widespread dispersion around the prototype brain structure (i.e., heterogeneity) is highly likely.44 This is because the process of adaptation in complex systems includes not only ordered transitions (homeostatis) but also a more chaotic, exploratory search trajectory when demands are excessive or repetitive, pushing the system beyond a critical point (allostasis).45 A detailed discussion of the brain’s complex adaptive dynamics is out of scope of this editorial (and has been addressed elsewhere46,47), but several lines of evidence lend support for the presence of higher allosatic load48 and the breakdown of complex neural dynamics in schizophrenia.49,50 Invoking complex adaptive systems in this context also helps to explain bidirectional adaptation; when disease propensity introduces supranormal biophysical properties (e.g., hyperconnectivity or hyperactivity in functional MRI), the compensating changes may be in the opposite direction (i.e., infranormal functions).
Operationalizing compensation
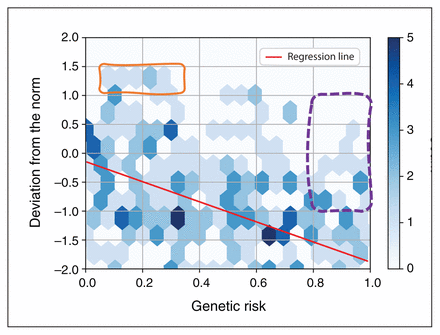
The idea of compensatory brain adaptation is well established in developmental psychopathology51 and aging neuroscience,52 but is not generally invoked when interpreting biological heterogeneity in schizophrenia (barring a few exceptions53). To date, it is unknown how the brain as a system compensates for physiologic deficits in this illness. In part, this speaks to the challenge of defining and operationalizing the concept of compensation in schizophrenia. Ideally, to define a neuroimaging observation as a marker of compensation, it should correct a known defect, be of benefit to the bearer (e.g., enhance cognition or reduce a symptom) and scale up and occur after an increase in demands on the system.52 These features can be conclusively shown only through longitudinal observations in patients with good prognostic trajectories who are ideally not on medications that can alter brain structure. Paradoxically, such patients cannot be found in long-term care settings and, thus, are seldom included in routine MRI studies.54,55 Nevertheless, it is important to note that superior functioning or supranormal structure or brain function alone cannot be indicative of compensation.51 The degree of compensation is best quantified by accounting for the severity of the adverse influence (i.e., the genetic risk) operating on an individual (e.g., Ioannidis and colleagues56 on measuring resilience during development). In cross-sectional studies, compensation can be operationalized as proximity to normative brain structure (and function) compared with others with a similar degree of adverse influence (e.g., higher polygenic risk scores). Compensation is much more than crude, subpersonal changes in biophysical properties. It is best understood as a dynamic process rather than discrete outcome. Nevertheless, as illustrated in Figure 1, using a formalism to define it may enable identification of a sufficient number of participants with putative compensated schizophrenia for further study. Without such practical steps toward studying noncausal phenomena, heterogeneity may remain unsolvable.57 Importantly, not all adaptive changes result in positive outcomes; chronic stress is known to induce maladaptation. Therefore, identifying a subgroup for further study, as proposed here, will be important to differentiate adaptation that is not beneficial from compensation (i.e., ameliorative adaptation).
A hypothetical hexplot (i.e., scattergram with counts of individuals, represented using hexagons with varying shades) indicating the negative correlation between polygenic risk (0 to 1) and normative deviation in brain structure (−2.0 to 2.0, z units). If compensated people are defined as those with supranormal brain structure, very few can be identified within our clinical samples (orange box). Alternatively, defining compensated schizophrenia as those with higher risk, but exhibiting low or no infranormal deviation, can identify larger numbers (purple dotted box). Here, supranormal values are assumed to represent compensation, given the example of polygenic risk score’s effect on brain volume, which is one of negative correlation. For some biophysical measures, notable infranormality is an index of a compensatory phenomenon (e.g., reduced hyperactivity). In this hypothetical plot, genetic risk can be replaced by environmental risk or medication exposure (e.g., when considering adverse effects), and the deviation from the norm can be for any biopsychosocial measurement (e.g., patient-reported outcomes). This synthetic plot was generated using https://jupyter.org.
Conclusion
As Vygotsky put it, not all deviations from the norm are signs of illness; in the case of schizophrenia, some may represent an adaptive response. Vygotsky further pushes us to see any defect as “stimuli for compensatory process.”1 Although the constitutional forces at play in schizophrenia contribute to deviations from the norm, the adaptive nature of the brain’s response highlights the role of compensation in brain heterogeneity. Compensatory processes are reactive and not causal; however, they can be shaped at an individual level if the biochemical, molecular and psychological determinants of it are understood. Doing so could open new therapeutic avenues in schizophrenia.
Footnotes
The views expressed in this editorial are those of the author and do not necessarily reflect the position of the Canadian Medical Association or its subsidiaries, the journal’s editorial board or the Canadian College of Neuropsychopharmacology.
Competing interests: Lena Palaniyappan reports personal fees from Janssen Canada, Otsuka Canada, SPMM Course Limited UK and the Canadian Psychiatric Association; book royalties from Oxford University Press; and investigator-initiated educational grants from Sunovion, Janssen Canada and Otsuka Canada, outside the submitted work.
Funding: Lena Palaniyappan’s research is supported by the Canada First Research Excellence Fund, awarded to the Healthy Brains, Healthy Lives initiative at McGill University (through a New Investigator Supplement to Lena Palaniyappan) and the Monique H. Bourgeois Chair in Developmental Disorders. He receives a salary award from the Fonds de recherche du Québec - Santé (FRQS).
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/