Abstract
Background: Childhood maltreatment has been found to play a crucial role in the development of psychiatric disorders. However, whether childhood maltreatment is associated with structural brain changes described for major depressive disorder (MDD) is still a matter of debate. The aim of this study was to investigate whether patients with MDD and a history of childhood maltreatment display more structural changes than patients without childhood maltreatment or healthy controls.
Methods: Patients with MDD and healthy controls with and without childhood maltreatment experience were investigated using high-resolution magnetic resonance imaging (MRI), and data were analyzed using voxel-based morphometry.
Results: We studied 37 patients with MDD and 46 controls. Grey matter volume was significantly decreased in the hippocampus and significantly increased in the dorsomedial prefrontal cortex (DMPFC) and the orbitofrontal cortex (OFC) in participants who had experienced childhood maltreatment compared with those who had not. Patients displayed smaller left OFC and left DMPFC volumes than controls. No significant difference in hippocampal volume was evident between patients with MDD and healthy controls. In regression analyses, despite effects from depression, age and sex on the DMPFC, OFC and hippocampus, childhood maltreatment was found to independently affect these regions.
Limitations: The retrospective assessment of childhood maltreatment; the natural problem that patients experienced more childhood maltreatment than controls; and the restrictions, owing to sample size, to investigating higher order interactions among factors are discussed as limitations.
Conclusion: These results suggest that early childhood maltreatment is associated with brain structural changes irrespective of sex, age and a history of depression. Thus, the study highlights the importance of childhood maltreatment when investigating brain structures.
Introduction
Previous studies have suggested that a relationship exists between childhood maltreatment and an increased risk for a number of mental disorders developing in adulthood,1,2 including major depressive disorder (MDD),3 posttraumatic stress disorder (PTSD),4 anxiety disorders2 and substance abuse.5 Depression, being one of the most severe mental disorders with a high prevalence of 15% in the general population, is of serious concern.6
Magnetic resonance imaging (MRI) has been extensively used to investigate the effects of MDD on brain structure, and it has been suggested that depression can independently cause morphological changes in several brain regions, including the anterior cingulate cortex (ACC), prefrontal cortices, hippocampus, caudate nucleus and putamen.7 A recent meta-analysis revealed that patients with MDD demonstrated hippocampal volumes 4%–6% smaller than matched controls.8 Furthermore, a new meta-analysis of structural imaging studies of depression confirmed volume reductions of the hippocampus, orbitofrontal cortex (OFC), caudate nucleus, putamen, globus pallidus and gyrus rectus volume in patients with MDD compared with healthy controls.9 Moreover, a bad clinical outcome with more relapses and a chronic course has been found to be associated with hippocampal, amygdala, ACC and dorsomedial prefrontal cortex (DMPFC) volume decline.10 However, growing evidence indicates that childhood maltreatment, defined as maltreatment or trauma in the form of emotional, physical or sexual abuse or emotional or physical neglect, could also have detrimental regional effects on brain structure that may provide vulnerability for the development of psychiatric disorders, including MDD.
Vythilingam and colleagues11 compared 32 women with re-current unipolar depression and prepubertal physical or sexual abuse to 11 women with depression but without prepubertal abuse and to 14 healthy controls. They found left hippocampal volumes to be 18% smaller in women with depression and prepubertal abuse than in women with depression but without abuse and 15% smaller than in healthy controls. Emotional neglect has also been associated with smaller hippocampal volumes. Smaller left hippocampal white matter volumes were reported in patients with MDD who had experienced emotional childhood neglect than in those without neglect. Both emotional neglect and brain structural abnormalities predicted cumulative illness duration.12 In addition, smaller left DMPFC volumes have been reported in 84 healthy controls and patients with MDD with a history of emotional maltreatment than in 96 comparison participants without maltreatment.13 Interestingly, this is in contrast to an experimental study by Spinelli and colleagues14 showing that compared with mother-reared monkeys, peer-reared monkeys withdrawn from their mother for 6 months had an enlarged vermis, DMPFC and dorsal ACC. It was suggested that these regions might be particularly “stress sensitive” and therefore vulnerable to structural morphological changes that may lead to an increased susceptibility to the development of psychiatric disorders.14
Recent studies in healthy participants and community samples have added to the evidence indicating that childhood maltreatment is associated with morphological brain alterations. In a large study of young adults with and without childhood maltreatment (n = 193), a reduction in the left hippocampal subfields CA2-CA3 and CA4-DG, CA1 and the subiculum was revealed in those with childhood maltreatment, irrespective of histories of MDD or PTSD.15 In 148 healthy participants, reduced grey matter volumes in the hippocampus, insula, OFC, ACC and caudate were found to be associated with high scores of childhood maltreatment. This association was not influenced by trait anxiety, depression level, age, intelligence, education or more recent stressful life events.16
Magnetic resonance spectroscopy has also been used to investigate the effects of childhood maltreatment. A single-voxel spectroscopy study found that the ratio of N-acetylaspartate to creatine was significantly lower in the ACC in 11 maltreated participants with PTSD than in 11 participants without maltreatment.17 In addition, a study of 18 maltreated children, compared with 20 nonmaltreated children, showed reduced grey matter in the medial OFC and the left middle temporal gyrus in those with maltreatment.18 Thus, these results suggested the OFC and ACC should be included in addition to the hippocampus and DMPFC as regions of interest for the present study.
To date, most studies have been conducted cross-sectionally in adults. A longitudinal MRI study involving 15 children aged 7–13 years with childhood maltreatment and PTSD reported that the presence of childhood maltreatment was related to a decrease of hippocampal volumes over a 12- to 18-month interval. However, this study did not have a comparison group of nonmaltreated individuals, hence no definite conclusion could be drawn about whether children with childhood maltreatment were more vulnerable for hippocampal changes over time.19 In contrast, maltreatment leading to childhood PTSD has been reported to result in larger hippocampal volumes in comparison to matched healthy, nonmaltreated children.20 Therefore, there is still debate on how and whether childhood maltreatment affects structural brain morphology. There is some evidence from previous studies in support of both childhood maltreatment and depression21–23 having effects on brain structure. However, it is less clear whether these changes are a result of childhood maltreatment or depression.
Preclinical studies have previously shown that childhood maltreatment can result in morphological changes of brain structure.14,24 It is thought that early-life stress can have detrimental neurobiological effects, including disturbances in processes such as synaptic production and elimination (pruning)25 and hypothalamus–pituitary–adrenal (HPA) axis regulation.26–28 It has also been suggested that early-life stress can cause inappropriate stimulation of neurotransmitters, neuroendocrine hormones and neurotrophins essential for normal brain development29 and that it may lead to vulnerability for the development of MDD.30 Since the hippocampus plays an essential role in emotion processing, behaviour and memory,31 the ACC in emotion and cognitive function,32 and the prefrontal cortex (frontopolar regions) in executive functions like decision-making,33 structural changes to these regions can disrupt these functions and thus the psychological state of a person; therefore, it is implicit to study them in the context of MDD and childhood maltreatment.
Using MRI and voxel-based morphometry (VBM), we aimed to investigate how and whether childhood maltreatment, current depression or both affect brain structure. Based on previous studies, we expected to see smaller hippocampal volumes as a result of childhood maltreatment, but there were inconsistent findings in previous studies with regards to ACC, OFC and DMPFC volumes. Moreover, we expected to see a more prominent decrease of hippocampal volume in patients with MDD with a history of childhood maltreatment. To further clarify whether there is also an independent effect of childhood maltreatment from diagnosis, we performed a secondary regression analysis with childhood maltreatment as a dimensional variable and with diagnosis.
Methods
Participants
We recruited currently depressed patients and healthy controls between the ages of 18 and 65 years from the Dublin South-West Mental Health Service. Some had experienced childhood maltreatment and some had not. Patients were recruited in our clinics by consultants of psychiatry and a research senior registrar. Thus, all patients were known to us and were already in treatment in the service. The exclusion criteria were history of neurologic or severe internal disorders, head injury or substance abuse. Healthy controls never experienced any psychiatric disorder (Axis I or Axis II), whereas patients were excluded in the case of any other comorbid psychiatry Axis I or Axis II disorder. Patients were allowed to receive an antidepressant monotherapy. Self- and observer-rated scales were also filled out for all participants.
Assessments
We used the Childhood Trauma Questionnaire (CTQ)34 to assess maltreatment experienced as a child or teenager. This questionnaire is a self-reported inventory that screens for 5 different types of childhood maltreatment: emotional, physical and sexual abuse, and emotional and physical neglect. It includes 28 items to be answered using a 5-point Likert scale ranging from “never true” to “very often true.” Early-life adversity is seen to be present when at least 1 of the subscales is above the threshold (emotional neglect > 14, physical neglect > 9, emotional abuse > 12, physical abuse > 9, sexual abuse > 7).34
The rating scales used were the 21-item version of the Hamilton Rating Scale for Depression (HAM-D),35 the Beck Depression Inventory (BDI-II)36 and the Structured Clinical Interviews for DSM-IV for psychiatric diseases (SCID-I37) and for personality assessment (SCID-II38). We obtained written informed consent from all participants after we provided them with a detailed description of the study, which was designed and performed in accordance with the ethical standards laid out by the Declaration of Helsinki. The ethics committee of Adelaide and Meath Hospital, incorporating the National Children’s Hospital and St. James’s Hospital, approved our study protocol.
MRI data acquisition
We obtained MRI scans with a Philips Achieva scanner (Philips Medical Systems) operating at 3 T. We used a sagittal T1 3-dimensional turbo field echo to scan all participants (repetition time 8.5 ms, echo time 3.9 ms, total acquisition time 7 min, number of acquisitions 1, field of view 256 × 256 × 160 mm, matrix of 256 × 256). All data sets were realigned to the anterior commissure.
Voxel-based morphometry
After manually reorienting and centreing the images to the anterior commissure, data preprocessing was performed using the SPM8 software package (Wellcome Department of Cognitive Neurology) running under MATLAB 6.5 (The MathWorks). We used the VBM8 toolbox, which entails a unified segmentation approach. It provides a generative model of VBM preprocessing that integrates tissue classification, image registration and MRI inhomogeneous bias correction. Thus, this advanced model avoids the circularity problem of the optimized VBM procedure, as the initial image registration does not require initial tissue segmentation and vice versa.39 For a detailed description, see http://dbm.neuro.uni-jena.de/467/. An important extension to the SPM8 segmentation is the integration of the Dartel normalization40 into the toolbox using an already existing Dartel template in Montreal Neurological Institute (MNI) space. Finally, the modulated grey matter partitions were smoothed with a 10 mm full-width at half-maximum Gaussian kernel and used for statistical analysis.
Statistical analysis
We used analysis of variance and χ2 tests to compare demographic variables among the 4 groups: patients with MDD and childhood maltreatment, patients with MDD without childhood maltreatment, healthy controls with childhood maltreatment and controls without childhood maltreatment.
We used a 2 × 2 factorial design to test for the interaction between childhood maltreatment (no history v. history of maltreatment) and diagnosis (MDD v. control) on grey or white matter volume. Age and sex were entered as covariates of no interest in the statistical design. Furthermore, regions of interest (ROIs) were defined with the intention to test grey and white matter volume differences in the hippocampus, ACC, dorsolateral prefrontal cortex (DLPFC), DMPFC and OFC. We identified ROIs using the Wake Forest University PickAtlas Toolbox, version 2.0,41 which provides a method for generating ROI masks based on the Talairach Daemon database. For ROI analyses, we corrected for multiple comparisons as follows: for ROIs chosen a priori, this was corrected for multiple voxel comparisons within this ROI (p < 0.05, family-wise error [FWE]-corrected). In addition, with respect to other brain regions, we also tested results at the whole brain level using voxel statistics (p < 0.05, FWE-corrected). We used a cluster threshold of 15 voxels to exclude results with very small clusters. A threshold of p < 0.001, uncorrected, with a spatial extent threshold of 15 contiguous voxels was also reported for the whole brain analysis. Coordinates of peak significant voxels were assigned to anatomic regions by means of anatomic labelling.42
For secondary analyses, we used stepwise regression analysis to investigate whether there was a further effect of childhood maltreatment in addition to the effect of depression diagnosis, age and sex. For this we extracted the grey matter volumes of the left and right hippocampus, OFC and DMPFC using MarsBaR (marsbar.sourceforge.net/). We used these brain volumes as dependent factors and diagnosis, age and sex as independent factors in a first step, and we used childhood maltreatment in a second step.
Results
Demographics and clinical variables
We enrolled 37 depressed patients and 46 controls in our study. Of the total 83 participants, 30 (20 patients, 10 controls) had experienced childhood maltreatment and 53 (17 patients, 36 controls) had never experienced childhood maltreatment. The demographic data as well as information on maltreatment and clinical data are shown in Table 1.
Demographic and clinical characteristics of patients with major depressive disorder and healthy controls with and without childhood maltreatment
The participants in each group were similar in terms of sex, height, weight and alcohol consumption. Although patients with MDD did not differ from controls with regards to age, controls without childhood maltreatment were younger than patients with MDD without childhood maltreatment (t = 2.1, p = 0.040) and controls with childhood maltreatment (t = 2.6, p = 0.013). Thus, age was used as a covariate in all analysis. The HAM-D, BDI and MADRS scores were significantly different between patients with MDD and controls, but no significant differences were evident between patients with MDD with and without maltreatment or controls with and without maltreatment. In addition, no significant difference was observed between the MDD groups with and without childhood maltreatment in age of onset, illness duration or number of days treated and untreated. Patients with MDD reported significantly more childhood maltreatment than controls and had significantly higher scores in emotional and physical neglect and total CTQ scores than participants in the other 3 groups. Controls with childhood maltreatment also had higher scores in physical and emotional neglect and higher total scores than controls without childhood maltreatment. Patients with MDD with childhood maltreatment had significantly higher scores in physical, emotional and sexual abuse than controls with childhood maltreatment and participants without childhood maltreatment (per definition; Table 1).
Regarding medication status, 13 patients were not taking antidepressants at the time of scanning, 12 patients were taking a serotonin reuptake inhibitor and 12 were taking a dual acting substance, such as venlafaxine or mirtazapine. None were taking tricyclic antidepressants. In addition to antidepressants 1 patient was taking 50 mg of quetiapine at night, 1 patient was taking 200 mg of quetiapine at night, and 1 patient was taking 1 mg of risperidone at night.
Analysis of the hippocampus, amygdala, DLPFC, DMPFC and ACC
Grey matter
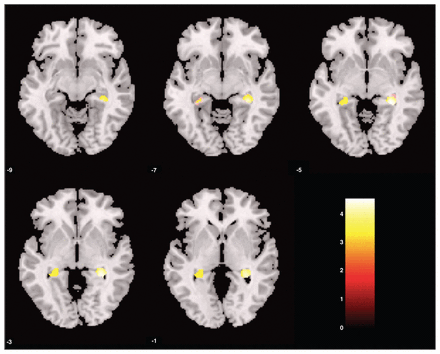
We observed an overall significant effect of childhood maltreatment, with smaller left and right hippocampal volumes evident in patients and controls who experienced childhood maltreatment than in those who did not (Table 2 and Fig. 1). Interestingly, participants who experienced childhood maltreatment also displayed significantly larger right DMPFC volumes and a trend toward significantly larger left OFC volumes than participants without childhood maltreatment.
Small volume–corrected grey matter density of participants with childhood trauma versus those without childhood trauma. Bilateral regions of the hippocampus were significantly smaller in participants with childhood maltreatment than those without. Statistical parametric maps were thresholded at p < 0.001, uncorrected, with a spatial extent threshold of 15 contiguous voxels. This was corrected for multiple voxel comparisons within the regions of interest (p < 0.05, family-wise error–corrected). The colour of the bar scales indicates the level of significance.
Voxel-based morphometry analysis of grey matter region of interest* volumes
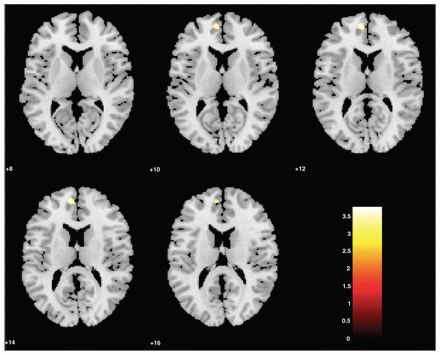
There was a main effect for the diagnostic group: patients with MDD had smaller left DMPFC and left inferior OFC volumes than controls (Fig. 2). There was no significant interactive effect between childhood maltreatment and diagnostic group (MDD, control). Nevertheless, patients without childhood maltreatment had smaller right OFC and trends toward significantly smaller left OFC and left ACC volumes than healthy controls without childhood maltreatment. Patients with childhood maltreatment had smaller hippocampal volumes than patients without childhood maltreatment (Table 2).
(Control > major depressive disorder [MDD]) Small volume–corrected grey matter density of healthy controls versus patients with MDD. The left regions of the dorsomedial prefrontal cortex were significantly smaller in patients with MDD than healthy controls. Statistical parametric maps were thresholded at p < 0.001, uncorrected, with a spatial extent threshold of 15 contiguous voxels. This was corrected for multiple voxel comparisons within regions of interest (p < 0.05, family-wise error–corrected). The colour of the bar scales indicates the level of significance.
White matter
Our VBM analysis did not reveal a significant main effect of childhood maltreatment on the white matter ROIs. However, the analysis did reveal an effect of diagnosis, with smaller white matter volumes in the left DMPFC in patients with MDD than in controls (cluster: pFWE = 0.024, K = 41, voxel: t = 3.72, MNI space x, y, z = −4, 50, 16). We detected no significant interactive effect between childhood maltreatment and the diagnostic group (MDD, control).
Whole brain analysis
The results of our grey and white matter whole brain analyses are depicted in the Appendix (available at cma.ca/jpn). On a whole brain analysis level, further regions reached significance (p < 0.001, uncorrected), but did not survive whole brain volume FWE-correction.
Regression analysis
Statistical information, in particular the statistics of change from regression analyses, are depicted in Table 3. While diagnosis of depression, age and sex explained 13% variance of the left hippocampus, childhood maltreatment also negatively affected hippocampal volumes and explained a further variance of 11.3%. For the right hippocampus, childhood maltreatment explained a further 15.4% of the variance, independent of the effect of age, sex and diagnosis. Within patients, older age and female sex was associated with smaller DMPFC volumes, whereas childhood maltreatment was related to increased DMPFC volumes. Left OFC volume was smaller in patients and older participants, whereas childhood maltreatment was independently associated with increased OFC volumes. Age, sex and diagnosis also influenced the right OFC, whereas childhood maltreatment did not exhibit a significant effect. There were no significant correlations between depression severity and brain volumes.
Results from the regression analysis*
Discussion
The results of the present study highlight the detrimental effects of childhood maltreatment on hippocampal development, and they are in agreement with those of previous studies showing reduced hippocampal volumes in participants who experienced early childhood maltreatment.11,12,15,16,23,43 Our study revealed that participants who experienced childhood maltreatment displayed significantly smaller right and left hippocampal grey matter volumes than participants without childhood maltreatment. Since no significant interactive effect was detected between childhood maltreatment and diagnosis, we conducted a secondary regression analysis to see whether the effects of childhood maltreatment are independent of age, sex and diagnosis of MDD. Childhood maltreatment significantly predicted reduced hippocampal volumes, even when the variance already explained by age, sex and diagnosis was taken out, highlighting the importance of childhood maltreatment on brain structure.
Reduced grey matter volumes in the hippocampus, insula, OFC, ACC and caudate have previously been found to be associated with high scores of childhood maltreatment, independent of trait anxiety, depression level, age, intelligence, education or more recent stressful life events in a healthy community sample.16 While we also found smaller hippocampal volumes to be associated with childhood maltreatment, we found significantly larger right DMPFC and left OFC grey matter volumes in participants with childhood maltreatment than in those without childhood maltreatment. Such increased DMPFC volumes have also been observed in peer-reared rhesus monkeys compared with mother-reared monkeys, demonstrating that in nonhuman primates childhood maltreatment can also cause abnormal morphological outcomes on brain development.43 However, this result is in contrast to that from a study showing smaller left DMPFC volumes in participants with a history of emotional maltreatment13 and to those from the previously mentioned study showing smaller OFC volumes.16
Our comparison of healthy controls and patients with MDD did not reveal any evidence of reduced hippocampal volume in patients. Similarly, a study in a different patient population did not find significant differences in hippocampal and amygdala volumes between patients with MDD and a control group.44 In contrast, recent meta-analyses and other studies, including previous studies by our group, found reduced hippocampal volumes in patients with MDD compared with controls.8,9,22,45 There is no obvious explanation for the negative finding of the present study, since we used similar inclusion and exclusion criteria as those used in other studies. Most likely it is due to the chance of a negative finding in biological studies with limited sample size. However, we found that patients with MDD had significantly reduced volumes in the left DMPFC and OFC compared with healthy controls. Interestingly, the finding of smaller left OFC volumes are in line with the results of a recent meta-analysis,9 which reported significantly smaller left but not right OFC volumes. Moreover, the finding of smaller left DMPFC volumes is also in agreement with the results of the meta-analysis, whereby we did not find smaller right DMPFC volumes. This result is also in line with those of our recent 3-year follow-up study,46 in which particular patients with a more chronic course of depression exhibited grey matter volume reductions in the DMPFC, ACC, hippocampus, DLPFC and OFC.46
Although left DMPFC volume was reduced in patients with MDD compared with controls, the right DMPFC volume was increased in participants with a history of childhood maltreatment compared with those without. The DMPFC is involved in events prediction, decision-making under uncertainty,47 emotion processing, emotional conflict evaluation48 and regulation of HPA responses.49 It has also been described as a nexus in the dysfunctional regulation of cognitive affective states.50 Hypoactivity of the right DMPFC has been observed in patients with remitted MDD compared with controls,51 suggesting that hypoactivity of this brain region may be associated with vulnerability to depression. Studies of healthy controls indicate that the DMPFC exerts executive inhibitory control over ventral limbic areas during effective reappraisal of negative stimuli,52 and this seems to suggest that increased DMPFC volumes might be a plastic consequence of the need for regulatory control in participants with childhood maltreatment. Unfortunately, to our knowledge, there is not much information available on the laterality of these functions.
With regards to the OFC, the left lateral OFC volume was reduced in patients with MDD compared with controls, whereas it was enlarged in participants with childhood maltreatment compared with those without childhood maltreatment. The lateral OFC is involved in inhibitory control of information processing as well as behavioural expression and is involved in emotion regulation. For example, Ochsner and colleagues53 found that the lateral OFC was more active when using reappraisal to decrease as opposed to increase negative affect.
These findings from previous studies could explain why participants with childhood maltreatment have increased DMPFC and OFC volumes and that these increases might be a necessary adaptive change due to childhood maltreatment, since they might need to regulate to a larger extent the emotional states associated with these negative experiences. However, this is speculative at this stage and needs further investigation.
Dysfunction in synaptic pruning or regulation of the HPA axis may also provide some explanation for these observed structural alterations. Synaptic pruning occurs in different brain regions as they mature at different stages, thus providing critical periods of region-specific brain development.54 Cognitive processes only mature after adolescence, therefore childhood maltreatment may impact this developmental path and may lead to inappropriate stimulation of neurotransmitters, neuroendocrine hormones and neurotrophins that are essential for normal brain development.29,54 The effect of childhood maltreatment on specific brain regions may be affected by age, severity and frequency of occurrence. As critical developmental periods exist, the vulnerability and reaction of the brain to childhood maltreatment may differ. Decreased hippocampal volume may represent atrophy as a result of childhood maltreatment, whereas increased OFC and DMPFC volumes may suggest some sort of compensatory mechanism. However, whether increased DMPFC and OFC volumes might reflect adaptations to early stress needs further investigation.
Limitations
It is important to note that there were some limitations to this study. First, the CTQ was completed retrospectively, which could lead to inaccurate recollection of events affecting CTQ scores. Investigating a study population that already had been investigated for childhood maltreatment during childhood might be able to overcome this issue. Second, the depressed group with a history of childhood maltreatment had higher CTQ scores than the controls with a history of childhood maltreatment. Thus, the groups weren’t matched for trauma exposure, and within the group of participants with childhood maltreatment even the differences between patients and controls may have been due in part to childhood maltreatment. However, we were able to overcome this issue in part by carrying out a secondary regression analysis using childhood maltreatment as a dimensional variable and thus taking into account all participants for the investigation of effects from childhood maltreatment. Third, the sample size used could have limited some of the results, particularly those involving the healthy controls with childhood maltreatment. For regions other than the hippocampus, amygdala, ACC, DLPFC and DMPFC, it is important to note that no voxels or clusters reached significance on whole brain analysis. The result of smaller hippocampal volumes associated with childhood maltreatment is in line with the results of many previous studies and highlights the importance of childhood maltreatment in brain development. Fourth, we found no significant interaction between diagnosis and childhood maltreatment. Thus, the results reported within the groups of patients with MDD and controls separately cannot be considered as robust and need further confirmation.
Conclusion
It has already been demonstrated that childhood maltreatment is associated with a more chronic course of MDD, especially with variables such as a longer duration of illness, earlier onset, greater number of episodes, suicidality and higher levels of dysfunctional attitudes and self-criticism.55 Although this was not an objective of the present study, in line with the results of previous reports56,57 we found a higher prevalence of childhood maltreatment among patients in whom MDD developed later in life than among healthy controls. Results from our previous longitudinal study on the effect of structural brain changes on depression outcome44,58 along with our present results indicate that childhood maltreatment may cause significant atrophy of the hippocampus, rendering the individual more susceptible to a more severe course of depression. The present results indicate that childhood maltreatment is associated with structural brain changes. The finding of reduced hippocampal volumes in participants with childhood maltreatment was confirmed in our study. Furthermore, we found that DMPFC and OFC volumes were enlarged in participants with childhood maltreatment. Whether an increase of PFC volumes could be related to changes in emotional regulation due to childhood maltreatment is speculative at this stage and needs to be confirmed and investigated further. However, childhood maltreatment seems to play a crucial role for regional brain volumes and needs to be considered in structural MRI studies.
Acknowledgements
We thank Science Foundation Ireland for a fund to conduct the present research granted to Thomas Frodl (SFI/07/SK/B1214C Science Foundation Strokes Professorship Grant) and Health Research Board Ireland for funding the research Centre of Advanced Medical Imaging.
Footnotes
Competing interests: As above for T. Frodl. None declared by any other authors.
Contributors: A. Carballedo, N. Skokauskas and T. Frodl designed the study. A. Chaney, A. Carballedo, A. Fagan, J. Meaney and T. Frodl acquired the data, which all authors except J. Meaney analyzed. A. Chaney, N. Skokauskas and T. Frodl wrote the article, which all authors reviewed and approved for publication.
- Received October 24, 2012.
- Revision received March 15, 2013.
- Accepted April 12, 2013.