Abstract
Background: The neurobiology of suicide is largely unknown. Studies of white matter tracts in patients with a history of suicidal behaviour have shown alteration in the left anterior limb of the internal capsule (ALIC). Our aim was to determine whether particular target fields of fibre projections through the ALIC are affected in depressed patients who recently attempted suicide.
Methods: We studied patients with major depressive disorder (MDD) with and without a history of suicide attempts and healthy controls using diffusion tensor imaging (DTI) and deterministic tractography to generate fibre tract maps for each participant. Tract voxels were coded as being unique to the left ALIC. We compared the mean percentage of fibres projecting to relevant brain regions in the 3 groups using analysis of covariance.
Results: We included 63 patients with MDD (23 with and 40 without a history of suicide attempts) and 46 controls in our study. Both groups of depressed patients had reduced fibre projections through the ALIC to the left medial frontal cortex, orbitofrontal cortex and thalamus. Those with a history of suicide attempts had greater abnormalities than those without suicide attempts in the left orbitofrontal cortex and thalamus.
Limitations: Diffusion tensor imaging deterministic tracking is unable to distinguish between afferent and efferent pathways, limiting our ability to distinguish the directionality of altered fibre tracts.
Conclusion: Frontothalamic loops passing through the ALIC are abnormal in patients with depression and significantly more abnormal in depressed patients with a history of suicide attempts than in those without a history of suicide attempts. Abnormal projections to the orbitofrontal cortex and thalamus may disrupt affective and cognitive functions to confer a heightened vulnerability for suicidal behaviour.
Introduction
Suicide is one of the leading causes of death in children and middle-aged individuals and represents a major public health problem throughout the world. Current trends suggest that about 1.5 million people will die by suicide in the year 2020.1 Major depressive disorder (MDD) is a highly prevalent, etiologically multifactorial and clinically heterogeneous disorder. The lifetime risk of suicide in patients with MDD is about 6%, which represents a 20-fold increase from the general population.2,3 Suicide attempts, defined as self-destructive acts with some degree of intent to end one’s life, are one of the strongest predictors of future suicidal acts.4,5 Thus, suicide attempts represent an important intervention point for suicide prevention efforts, and in vivo studies of individuals who attempt suicide represent an important strategy for learning about the neurobiology of suicide.
Conventional magnetic resonance imaging (MRI) studies have found white matter and grey matter hyperintensities and reduced orbitofrontal cortex grey matter volumes on scans of depressed patients with a history of suicide attempts.6–8 A functional MRI experiment revealed that suicidal patients were distinguished from nonsuicidal patients in their activation patterns in frontal, cingulate and cerebellar regions.9 A positron emission tomography study revealed localized prefrontal hypofunction and impaired responsivity of serotonergic systems to pharmacological challenge that were proportional to the lethality of the suicide attempt in individuals with MDD.10 A meta-analysis of white matter findings revealed specific decreases in the integrity of the superior longitudinal fasciculus in patients with MDD, implicating fibre pathways that connect parietotemporal association areas with the frontal lobe.11 Abnormalities in the anterior limb of the internal capsule (ALIC) have also been identified in patients with depression,12 and other data indicate that the ALIC is affected by early-life stress during development.13 These studies have increased the understanding of possible anatomic and functional substrates for suicidal behaviour, but the specific neural circuits implicated in suicide risk remain to be fully elucidated.
Diffusion tensor imaging (DTI) measures diffusion characteristics of water molecules in vivo to identify the direction and integrity of fibres within white matter14 and is a promising approach for investigating white matter pathways in neuropsychiatry. Diffusion tensor imaging tractography models fibre orientation based on neuroanatomically situated “seeds.”15 Deterministic white matter tractography uses streamline algorithms defining the local tract direction by the major eigenvector of the diffusion tensor. These approaches have been used to map out white matter pathways in the human brain noninvasively and are now commonly used in clinical applications.16
In a previous study, we found that patients with MDD who had a history of suicide attempts had more severe microstructural white matter alterations in the left ALIC than other depressed patients.12 Moreover, the ALIC has been reported to be involved in multiple psychiatric disorders, particularly schizophrenia,17,18 obsessive–compulsive disorder (OCD),19 and major depression.20 Furthermore, the ALIC is the most frequent therapeutic target when deep brain stimulation is used clinically for treatment-resistant depression and OCD.21 The microstructural alteration in ALIC white matter indicates an abnormality of fibre pathways passing through the ALIC in suicidal patients with MDD. To examine brain regions affected by this alteration in hypothesis-driven research, brain regions with reciprocal projections through the ALIC need to be identified and examined. In the present study, we performed structural connectivity analysis and used DTI-based deterministic tractography to characterize white matter fibre projections passing through the left ALIC and their potential alteration in patients with MDD who had a recent history of suicidal behaviour.
Methods
Participants
We recruited patients with MDD from the Department of Psychiatry in the West China Hospital of Sichuan University. They met the diagnostic criteria for MDD, as determined using the Structured Clinical Interview for DSM-IV (SCID).22 Patients were required to have no history of electroconvulsive therapy, to be medication-free for at least 2 weeks before the study and to have a Hamilton Rating Scale for Depression (HAM-D) total score of 18 or higher.23 To be included in the group of suicidal patients, participants had to have a history of at least 1 suicide attempt, defined as a self-destructive act with intent to die,24 within 1 month before magnetic resonance imaging. Conversely, to be included in the nonsuicidal group, they were required to have no such history in their lifetime. Patients were not considered to have a history of suicide attempts if they had only suicidal thoughts or a suicide plan, or if they had self-injurious behaviours without intent to die. Exclusion criteria were any other DSM-IV Axis I comorbidities; any current severe medical problems, including central nervous system disease; any history of severe head trauma; and substance or alcohol abuse or dependence within the 12 months preceding the study.
Healthy controls were recruited from the local area by poster advertisement and assessed using the nonpatient version of the SCID. Exclusion criteria were history or present diagnosis of any DSM-IV Axis I disorder, any neurologic illness, any history of head trauma with loss of consciousness, and any known history of psychiatric disorders or suicide among first-degree relatives.
The West China Hospital Clinical Trials and Biomedical Ethics Committee of Sichuan University approved our study protocol, and written informed consent was obtained from all participants.
Data acquisition
Images were acquired on a 3 T GE EXCITE MRI scanner (General Electric Medical Systems) using a single-shot echo planar imaging sequence with an 8-channel phased array head coil. Participants were fitted with soft ear plugs, positioned comfortably in the coil and instructed to relax and keep still. We minimized head motion using foam pads. For each slice, we collected 15 images with high diffusion-weighting along 15 noncollinear and noncoplanar directions: repetition time 10 000 ms, echo time 70.8 ms, slice thickness 3 mm, field of view 240 × 240, in-plane resolution 1 × 1 mm2, matrix 128 × 128, b 1000 s/mm2. This clinical protocol was selected to balance interest in completing scans quickly for our untreated acutely ill patients to minimize motion artifact, while providing sufficient resolution to detect effects of clinical interest.
Image processing
To reconstruct white matter fibres in the whole cerebral cortex, we followed 2 steps. First, head motion was removed by aligning all diffusion-weighted images to the unweighted S0 image with a b value of 0 s/mm2, and eddy current distortions were corrected by affine registration to the reference S0 image using DtiStudio v3.0.1 software (http://mri.kennedykrieger.org/sitemap/software.htm). Second, we used diagonalization of the diffusion tensor matrix to yield voxel-based eigenvalues and eigenvectors using Diffusion Toolkit software (v0.6.0; Massachusetts General Hospital), then voxel-based fractional anisotropy (FA) values were generated from these eigenvalues. Finally, we applied the streamline tracking algorithm to generate DTI tractography maps.25 Fibre bundles were reconstructed with Trackvis (v0.5.1, www.trackvis.org).26
Our 3-dimensional characterization of fibre tracts of interest using a continuous tracking (interpolated streamline) deterministic algorithm is a swift, easily interpretable method that is readily applicable to DTI acquisition streams.27 This kind of deterministic algorithm identifies the orientation of maximum diffusion at each voxel as the principal eigenvector. In this way the method follows the main fibre direction as revealed by the diffusion model, which uses sequences of points that follow white matter fibres28 to identify white matter tracts that are crucial in transferring information across nodes in neural systems.
Drawing and extracting regions of interest
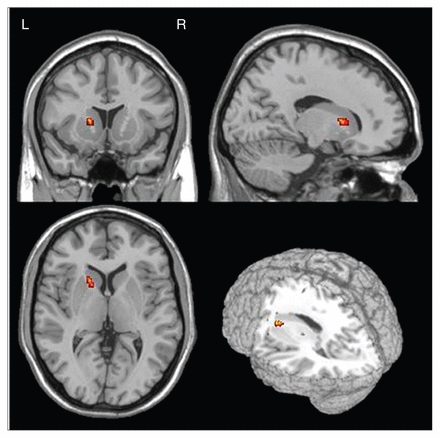
We chose the left ALIC as the seed region of interest (ROI) with its central Talairach coordinate, x, y, z = −18, 16, 1, based on findings from prior research (Fig. 1).12 The brain regions of fibres projecting through the left ALIC were extracted from a standard anatomic labelling (AAL) template.29
The left anterior limb of the internal capsule was chosen as the seed region of interest. Talairach coordinates x, y, z = −18, 16, 1) were converted in Montreal Neurological Institute space.
To extract the ALIC and its fibre projections for each participant, we followed 4 steps. First, each participant’s image with a b value of 0 s/mm2 was generated from each raw data set using Diffusion Toolkit software. Second, each participant’s T1-weighted image was coregistered to their b0 image using a linear transformation. Third, the coregistered T1 image was spatially normalized on the AAL template using a nonlinear transformation (T). Finally, ROI segmentations in the AAL template were warped to DTI native space using an inverse transformation (T−1). These steps were carried out in SPM8 software (Wellcome Trust Centre for Neuroimaging).
To quantify the integrity of fibres passing through the ALIC to areas to which it sends and receives projections, we calculated the mean percentage of fibres passing through the ALIC to encephalic ROIs. For each participant, this was defined as the percentage of fibres projecting to the ROIs (areas where patient–control differences were identified) that pass through the ALIC relative to the total numbers of fibres passing through the ALIC. We generated group level tract maps by including only those voxels common to 95% connectivity likelihood. The formula was as follows: pf = (rf ÷ tf) × 100%, where pf represents the percentage of fibres passing through the ALIC and projecting to a specific ROI, tf represents the total number of fibres passing through the ALIC and rf represents the number of identified fibres passing through the ALIC and projecting to a specific ROI. A higher percentage represents a greater relative proportion of fibres projecting to specific encephalic regions. The internal capsule passes between the caudate and putamen, with fibres projecting from both its anterolateral and posterior-medial aspects. The percentage of fibres projecting to different ROIs passing from the most posterior-medial aspect of the ALIC to its posterior target fields (including the thalamus) and the percentage of fibres passing anteriorly from the ALIC to the frontal cortex and other regions were computed separately. Thus, for example, the percentage of estimated fibres to the thalamus represents the percentage of fibres passing from the posterior aspect of the ALIC that reach the thalamus. We computed similar percentage estimates for projections to frontal lobe regions from the anterior aspect of the ALIC.
While fibre tracking with DTI evaluates white matter pathways, it is important to note that it is a relative measure and does not represent an absolute quantitative index of the actual number and condition of white matter fibre bundles. The difference between the DTI fibre estimate and the actual number of axonal fibres depends on several methodological factors, including the arbitrary undersampling of the DTI algorithm, FA threshold, crossing fibres, noise level, native resolution, axonal dimension and density.30
Statistical analysis
We compared the mean percentage of fibres traversing through the ALIC and projecting to specific encephalic regions in the 3 groups using analysis of covariance, with age, sex and illness duration as covariates. Post hoc between-group t tests were used to determine significant differences among the groups. Findings with 2-tailed tests were deemed significant at p < 0.05 after Bonferroni correction for multiple comparisons.
Results
Participants
All participants were of Chinese Han nationality. We enrolled 23 patients with MDD who had a history of a suicide attempts (15 women, 8 men, average age 36.3 ± 14.5 yr), 40 patients with MDD who had no history of suicide attempts (19 women, 21 men, average age 34.0 ± 14.5 yr), and 46 healthy controls (25 women, 21 men, average age 33.3 ± 11.4 yr). All participants were right-handed. This was a larger sample than we have described in our previous report:12 there were 7 more patients with a history of suicide attempts and 4 more patients without a history of suicide attempts.
The demographic and clinical characteristics of participants are described in Table 1. Patients who attempted suicide did not differ from those who did not attempt suidice in sex, age, education, illness duration or 17-item HAM-D scores (all p < 0.05). There were no significant differences between patients and controls in age, sex or education.
Demographic and clinical characteristics of patients with major depressive disorder with or without a history of suicide attempts and healthy controls
Connectivity of fibres traversing through the ALIC
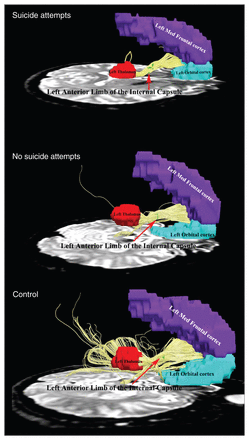
The left ALIC seed showed robust ipsilateral connections to the medial frontal cortex (MFC), orbitofrontal cortex (OFC), and left thalamus in patients with MDD and controls (Fig. 2).
Connectivity of fibres traversing through the left anterior limb of the internal capsule projected forward to the left medial frontal cortex and left orbitofrontal cortex and posteriorly to the left thalamus in depressed patients with a history of suicide attempts, those without a history of suicide attempts and healthy controls. The fibre pathways were significantly different among the 3 groups.
Omnibus test of group differences
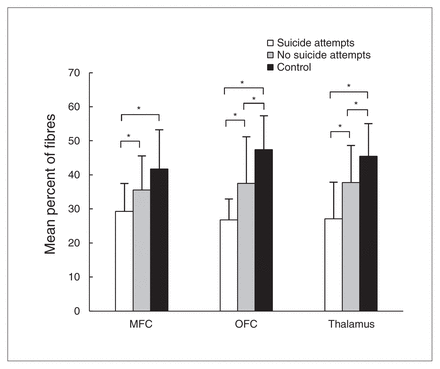
In patients with MDD who had a history of suicide attempts, the mean percentage of identified fibres projecting to the left MFC was 29.3 ± 8.25, to the left OFC was 26.8 ± 6.13 and to the left thalamus was 27.1 ± 10.8. In patients with MDD who did not have a history of suicide attempts, the mean percentage of fibres projecting to the left MFC was 35.6 ± 10.0, to the left OFC was 37.5 ± 13.7 and to the left thalamus was 37.8 ± 10.9. In controls, the mean percentage of fibres projecting to those regions was 41.7 ± 11.5, 47.4 ± 9.98 and 45.5 ± 9.62, respectively. The 3 groups differed in the mean percentage of fibres in left MFC (F = 6.039, p = 0.004), left OFC (F = 12.735, p = 0.001) and left thalamus (F = 11.107, p = 0.001; Table 2 and Fig. 3).
Mean percentage of anterior limb of the internal capsule (ALIC) fibres differed significantly among the 3 groups projecting to left medial frontal cortex (MFC), left orbitofrontal cortex (OFC) and left thalamus (all p < 0.005). In the left OFC and thalamus, patients both with and without a history of suicide attempts showed lower mean percentages of fibres projecting from the ALIC than healthy controls (both p < 0.05), and those with a history of suicide attempts showed lower mean percentages of fibres projecting from the ALIC than those without a history of suicide attempts in these 2 regions (both p < 0.01). In left MFC, patients both with and without a history of suicide attempts had significantly reduced fibres projecting from the ALIC relative to healthy controls (both p < 0.05), but no significant difference was found between the 2 patient groups in this region. *p < 0.05, 2-tailed.
Mean percentage of fibres projecting from the ALIC to the left MFC, OFC and thalamus in patients with MDD with and without a history of suicide attempts and healthy controls
Pairwise group comparisons of fibre pathways
Patients both with and without a history of suicide attempts showed lower mean percentages of fibres connecting the ALIC to the left MFC than healthy controls (p = 0.001 and p = 0.049, respectively), yet we found no significant difference between patients with and without a history of suicide attempts in this region (p = 0.10; Table 2 and Fig. 3).
In the left OFC, patients both with and without a history of suicide attempts showed lower percentages of projecting fibres than healthy controls (p = 0.001 and p = 0.006, respectively). Patients with a history of suicide attempts showed significantly greater reductions in the percentage of projecting fibres than those without a history of suicide attempts in this region (p = 0.009; Table 2 and Fig. 3).
In the left thalamus, patients both with and without a history of suicide attempts showed lower percentages of projecting fibres than healthy controls (p = 0.001 and p = 0.022, respectively), and patients with a history of suicide attempts showed lower percentages of projecting fibres than those without a history of suidice attempts in this region (p = 0.006; Table 2 and Fig. 3).
We found no association between duration of illness or HAM-D scores and the mean percentage of fibres projecting from the left ALIC to the left MFC, OFC and left thalamus in patients with or without a history of suicide attempts.
Tracking numbers of fibre projection
The DTI fibre tracking estimate of the number of fibres projecting to left MFC, OFC and left thalamus and of fibres traversing through the left ALIC in all 3 groups are presented in the Appendix, Figure S1 and Table S1, available at jpn.ca. The estimated number of fibres to the 3 target ROIs follows a pattern of suicide attempts < no suicide attempts < control (all p < 0.01). We found no significant group differences in the total estimated number of fibres traversing through the left ALIC (p = 0.20).
Mean FA values of fibres
Mean FA values of fibres projecting to the left MFC, OFC and left thalamus and total fibres traversing through the left ALIC in all 3 groups are presented in the Appendix, Figure S2 and Table S2. There are significant differences in mean FA values among the 3 groups in the ALIC itself and in the left ALIC fibres projecting to the MFC, OFC and thalamus (all p < 0.05).
Discussion
In this study, we used DTI tractography to examine the anatomy of a specific white matter pathway (left ALIC) where alterations have been associated previously with suicidal behaviour in depressed patients in terms of its projections to specific brain regions that receive and send projections through the internal capsule. The main findings were abnormalities in fibre connections passing though the left ALIC projecting to the left MFC and OFC and posteriorly to the left thalamus in depressed patients with and without history of suicide attempts. Depressed patients with a history of suicide attempts showed a significantly greater reduction in fibres linking the ALIC to left OFC and thalamus than patients without a history of suicide attempts.
The ALIC is the white matter tract in front of the genu and between the head of the caudate nucleus and the lenticular nucleus that contains both ascending and descending axons. In a previous study, we found that patients with a history of suicide attempts had lower FA in the left ALIC than patients without a history of suicide attempts and healthy controls.12 We conducted the present study to clarify the particular target fields of the ALIC to which projections are altered in patients with MDD with a history of suicide attempts relative to depressed patients without a history of suicide attempts. The results support the hypothesis that depressed patients with a history of suicide attempts had white matter impairments in specific frontothalamic pathways compared not only with healthy controls but also with patients without a history of suicide attempts. These findings may help reveal neurobiological mechanisms of suicide with regard to the anatomic integrity of specific nerve fibre pathways.
The frontothalamic loop and suicidal behaviours in depressed patients
Our findings using in vivo DTI studies describing the anatomy of fibre pathways projecting through the ALIC are highly consistent with those of previous histological studies showing connections to the prefrontal cortex (PFC; including the MFC and OFC) and thalamus.31–34 This reflects the power and promise of DTI for testing clinical hypotheses, as posed in this study. In vivo structural and functional imaging studies, as well as postmortem investigations of adults, suggest that the frontal–striatal–thalamic and limbic–thalamic–frontal neural circuits have an important role in the pathogenesis of depression.35,36 Our data provide new detail regarding specific alterations in frontothalamic loops, where anatomic abnormalities in thalamocortical and corticothalamic pathways may contribute to functional disruption of neural circuits that influence behavioural control and emotion processing in ways that increase the risk for suicidal behaviour in depressed patients.37,38
The impairment of frontothalamic circuitry in depressed patients with a history of suicide attempts
In the present study, the percentage of fibres connecting the MFC and OFC to the anterolateral aspect of the ALIC and connecting the ALIC and thalamus were decreased in depressed patients with a history of suicide attempts compared with patients without a history of suicide attempts and controls. These alterations in fibres connecting the ALIC and the 3 target ROIs resulted primarily from reductions in fibres passing to a specific ROI rather than from an overall reduction in the number of fibres passing through the ALIC, which did not differ among the groups (Appendix, Figure S1 and Table S1). The first of these effects may reflect intrinsic impairment in the MFC and OFC. These regions are anatomically defined as the PFC regions that receive robust projections from the magnocellular, medial nucleus of the mediodorsal thalamus.39 Impaired performance on cognitive tasks and executive functions as well as a reduced ability to modulate emotional responsivity have been linked to a history of suicidal behaviours.40 In previous studies, changes in brain structure and function in individuals with a history of suicidal behaviour are mainly found in the OFC and MFC, where changes could cause neuropsychological disturbances in decision-making, problem solving and affect modulation, which have been reported in people with a history of suicide attempts and in depressed patients.41 The thalamus receives strong dopaminergic projections and plays a critical role in the mood-related neural networks.42 Previous research has shown that functional and histological abnormalities in the thalamus are involved in the pathophysiology of depression and suicidal behaviours.43
The present study also showed that depressed patients without a history of suicidal behaviour had decreased fibres in frontothalamic pathways passing to specific prefrontal regions and to the thalamus relative to healthy controls. In patients with a history of suicide attempts, we observed a more severe reduction in these pathways in the OFC and thalamus than in depressed patients without a history of suicide attempts. These particular alterations in frontothalamic pathways in patients with a history of suicide attempts may not only reflect abnormalities related to depression, but also abnormalities that are particularly relevant to factors related to suicide attempts. Clarification of whether reductions in these white matter pathways might lead to compensatory changes, such as an increase of projections to other brain regions, awaits further study. In any case, the pattern of neuroanatomic alterations in a neural circuitry crucial for affect regulation in patients with a history of depression and suicidal behaviour merits further study to clarify both its anatomy and its functional implications.
Depression has been hypothesized to result in part from a maladaptive plasticity in response to external stressors.44 Applying this model, one might speculate that the microstructural abnormalities in projections that transfer signals from the OFC to the thalamus through the ALIC may disturb function in frontothalamic pathways, which could disrupt cognition and emotion processing. Such disturbances may confer risk for suicidal behaviour by either disinhibiting emotional responses and behaviour or by disrupting the ability to plan adaptive behavioural responses to stressful circumstances. The ALIC contains numerous afferent and efferent fibres running from the frontal lobes to the thalamus.45 Clinically, the ALIC is the most frequent therapeutic target in deep brain stimulation treatment for depression.46,47 Targeting the ALIC for this procedure is designed to disrupt activity in the loop fibres that connect the cortex with the thalamus to, in theory, disrupt abnormal functioning in that circuitry.47 Thus, clinically relevant alterations in the ALIC may contribute to affective and behavioural symptoms, including suicidal behaviour, and represent a target for therapeutic intervention. Alterations in the ALIC are common across multiple disorders where suicide risk is increased,17 so further research may indicate a relevance of the data presented here to suicide risk across multiple psychiatric disorders.
The reduction of fibre projections from the ALIC to the target fields of interest might be due to neurodevelopmental factors or pathological changes. It is also possible that an alteration in the white matter tracts may have made them less detectable by the DTI deterministic tracking method. This possibility is suggested by the reduced FA values of tracking fibres. The extent that there is an alteration of fibre integrity rather than loss of axonal projections, or influences of neural plasticity acquired from external environment, or genetic factors causing an actual change in the development of the fibre tracts of interest remains to be determined by future studies.
Limitations
There are several limitations to our study that merit consideration. First, the study did not have the power to examine variability in brain anatomy with regard to any particular method of suicide attempts, specific previous treatments, or number of previous attempts and depressive episodes, although these factors might in larger samples be found to be related to alterations in brain structure. Second, we didn’t formally evaluate personality, and because of the high rate of comorbid personality disorders in depressed patients who attempt suicide, it will be important in future studies to examine whether depressed patients with personality disorders and a history of suicidal behaviour have greater ALIC impairment. Third, DTI deterministic tracking is unable to distinguish between afferent and efferent pathways, nor is it informative with regard to the role of these projections in controlling excitatory and inhibitory processes in their target fields. Therefore the directionality of the observed frontothalamic connections cannot be inferred from our results, nor do they on their own suggest specific neurophysiological or neurochemical changes. These factors need to be examined in future studies in which the function as well as the anatomy of this circuitry are examined simultaneously. Nonetheless, the reproducibility of the DTI-based tractography method is well established, and the results generally match those of fibre tracing in nonhuman primates and humans in postmortem analysis.48 Fourth, the degree to which alterations in the ALIC are restricted to the left hemisphere needs to be evaluated in future studies. Finally, the present results cannot establish the direction of causality (i.e., whether decreased fibres projecting to the PFC and thalamus caused a predisposition to suicide attempts in depressed patients or vice versa, or even if both are the result of a separate cause). To clarify this, a longitudinal approach would be required to examine depressed patients before and after a suicide attempt.
Conclusion
The present study shows that white matter fibre abnormalities in frontothalamic circuits passing through the ALIC are abnormal in depressed patients with a history of suicide attempts. These alterations may contribute to cognitive and affective alterations and thereby increase vulnerability for suicidal behaviour in depressed patients. Future longitudinal studies are needed to explore the potential relationship between the impaired frontothalamic circuits and depression and suicidal behaviours in order to determine the utility of ALIC alterations as a predictor of risk for future attempts.
Acknowledgements
This study was supported by National Natural Science Foundation (Grant Nos. 81030027, 81271532, 30900378, 81227002 and 81220108013), National Key Technologies R&D Program of China (Program No: 12BAI01B03) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) of China. The authors want to acknowledge the American CMB Distinguished Professorship Award to Q. Gong and a Von Humboldt Research Award to J.A. Sweeney. Q. Gong also acknowledges his Visiting Adjunct Professor appointment in the Department of Radiology at the University of Illinois Hospital & Health Sciences System, Chicago (IL), USA.
Footnotes
Competing interests: J. Sweeney has received support from BMS, Roche, Lilly, Takeda and Janssen.
Contributors: Z. Jia and Q. Gong designed the study. X. Huang, W. Kuang, Q. Wu and S. Lui acquired the data, which Z. Jia, Y. Wang, W. Kuang, J.A. Sweeney and Q. Gong analyzed. Z. Jia, Y. Wang, J.A. Sweeney and Q. Gong wrote the article, which all authors reviewed and approved for publication.
- Received February 7, 2013.
- Revision received May 27, 2013.
- Revision received July 10, 2013.
- Accepted July 15, 2013.