Abstract
Background: Acute neural effects of antidepressant medication on emotion processing biases may provide the foundation on which clinical outcomes are based. Along with effects on positive and negative stimuli, acute effects on neutral stimuli may also relate to anti-depressant efficacy, yet these effects are still to be investigated. The present study therefore examined the impact of a single dose of the selective serotonin reuptake inhibitor escitalopram (20 mg) on positive, negative and neutral stimuli using pharmaco-fMRI.
Methods: Within a double-blind, randomized, placebo-controlled crossover design, healthy women completed 2 sessions of treatment administration and fMRI scanning separated by a 1-week washout period.
Results: We enrolled 36 women in our study. When participants were administered escitalopram relative to placebo, left amygdala activity was increased and right inferior frontal gyrus (IFG) activity was decreased during presentation of positive pictures (potentiation of positive emotion processing). In contrast, escitalopram was associated with decreased left amygdala and increased right IFG activity during presentation of negative pictures (attenuation of negative emotion processing). In addition, escitalopram decreased right IFG activity during the processing of neutral stimuli, akin to the effects on positive stimuli (decrease in negative appraisal).
Limitations: Although we used a women-only sample to reduce heterogeneity, our results may not generalize to men. Potential unblinding, which was related to the subjective occurrence of side effects, occurred in the study; however, manipulation check analyses demonstrated that results were not impacted.
Conclusion: These novel findings demonstrate that a single dose of the commonly prescribed escitalopram facilitates a positive information processing bias. These findings provide an important lead for better understanding effects of antidepressant medication.
Introduction
Affective disorders, such as major depressive disorder and generalized anxiety disorder, are characterized by a negative information processing bias whereby negative information is more prominent than positive information.1,2 Early neural effects of antidepressants on emotion processing biases may underpin downstream changes associated with improvement of symptoms in people with affective disorders.2–4 There is a critical need to better understand acute antidepressant action given low response and remission rates to first-line treatment, resulting in trial and error.5,6 Though it has been established that patients with affective disorders also negatively appraise neutral stimuli,7–9 investigations on the acute effects of antidepressants have yet to account for such effects on neutral stimuli. If treatment were to facilitate the processing of neutral stimuli — stimuli that patients usually perceive as negative — in addition to positive and negative stimuli, this would provide much-needed support for the proposal that treatment improves negative biases across stimuli by facilitating a positive information processing bias.2–4 In addition, previous findings on the acute effects of citalopram on emotion processing10,11 must be updated by focusing on the more commonly prescribed treatment, escitalopram.12 To these ends, the present study examined the acute impact of escitalopram on the processing of positive, negative and neutral images to lay the framework for better understanding early physiologic effects of current antidepressant treatment.
Affective disorders are related to impairment in monoamine neurotransmitter systems and intracellular processes,13 which are modulated by antidepressant treatment and lead to symptom improvement.13,14 The most commonly prescribed class of antidepressants is the selective serotonin reuptake inhibitor (SSRI). Through serotonergic innervation of brain regions linked to affective disorders,14,15 SSRIs act on brain regions responsible for the processing of emotional information.16,17 Our recent meta-analysis17 of the impact of antidepressants on emotion processing showed that SSRIs produce differential acute effects on regional activation that depend on the type of emotion stimuli used in the task. Single dose SSRI administration impacts on both emotional reactivity and on appraisal and regulatory processes during the processing of complex emotional scenes (e.g., International Affective Picture System [IAPS]18). The amygdala and inferior frontal gyrus (IFG) are key regions implicated in emotion reactivity and its appraisal and regulation, respectively,19,20 and are regions modulated by single dose SSRI administration.17 While the amygdala reliably and automatically21,22 responds to the salience of affective stimuli, the IFG exerts top–down executive control over appraisal and affective responses.23–25 Critically, the IFG plays an important role in attenuating amygdala activity in response to affective stimuli, facilitating effective appraisal and regulation.25–27 The reciprocal relationship between the IFG and the amygdala is apparent during the appraisal and reappraisal of emotional stimuli,25,27 highlighting the IFG and amygdala as key components in an emotional processing circuit. Depressed patients repeatedly demonstrate increased amygdala activation and reduced IFG activation, suggesting a lack of regulation on the part of the IFG over amygdala function.28,29 Indeed, single dose and subchronic SSRI treatment is associated with the normalization of activity in these areas in both healthy11,30,31 and clinical samples.32,33
While the precise biological mechanisms of antidepressants remain to be understood, recent theory suggests that anti-depressants act, starting at the first dose, by changing the way individuals process affective information.2–4 Cognitive neuro-psychological models suggest that these changes lead to processing environmental stimuli more positively and a relearning of affective associations, eventually leading to symptom improvement.2–4 In addition, while antidepressant response generally takes weeks before it is apparent clinically, an increasing body of work has examined the ability to predict response to antidepressant medications.5,34 Recent research10,11,35 has revealed that there are directly observable physiologic changes that occur within hours of administering a single dose, demonstrating that differential drug effects may be observed following acute rather than chronic administration of antidepressants before noticeable behavioural changes emerge.
Our earlier work using electroencephalography (EEG) demonstrated that 20 mg of citalopram potentiates cortical electrophysiological responses to positive emotional pictures and attenuates responses to negative emotional pictures.10 We36 and others2,4 have proposed that antidepressant-induced reductions in clinical symptoms may be associated with these early neurophysiological and neuropsychological responses during the processing of emotional stimuli and that these changes may be observed. Given EEG’s lack of spatial resolution for subcortical regions of interest (ROI; namely, the amygdala), pharmaco-fMRI studies are required in order to further examine the neurophysiology of regions involving both reappraisal (e.g., IFG) and reactivity (e.g., amygdala) during acute antidepressant treatment, reflecting shifts from negatively biased to more positively biased appraisal and reactivity responses. Acute pharmaco-fMRI studies11,31,37–41 have provided additional insight into the responses of emotion circuitry and emotion bias as a treatment target for SSRIs. However, examinations have yet to challenge the emotion circuitry with complex images ranging in emotional content to evoke differential cognitive appraisal processes rather than simple emotional facial expressions that elicit automatic processes related to basic emotional salience.42,43 Specifically, studies have yet to examine the effect of acute anti-depressant treatment on neutral stimuli. This is an important consideration given that patients with affective disorders28,29,44 display impairments in the conscious and controlled processing of both emotional45,46 and neutral stimuli.7–9 Rather than consider the impact of acute effects of antidepressants on neutral stimuli as a condition of interest in its own right, treatment studies thus far have employed neutral stimuli to serve as a contrast against positive and negative emotional stimuli to isolate modulations in neural activity due to emotion.10,11,31 Antidepressant treatment may not modulate amygdala activity associated with reactivity to low-arousal neutral stimuli; however, IFG activity associated with appraisal and regulation may be impacted. If treatment positively biases the appraisal of neutral stimuli, it could be expected that treatment impacts IFG activity in a similar way as positive stimuli. Finally, escitalopram was selected for study given increasing prescribing rates12 and its improved efficacy over citalopram,47,48 which may have a neural explanation.49 Furthermore, pharmaco-fMRI study of acute treatment effects on emotional processing across differently valent categories is required to further cognitive neuropsychological models of anti-depressant action.
Here we examine the acute impact of escitalopram administration on neural responses to highly arousing positive and negative pictures as well as low-arousal neutral pictures in healthy participants. Consistent with and building on our previous findings,10 we predicted that the impact of escitalopram on amygdala and IFG blood oxygen level–dependent (BOLD) response would depend on emotional valence such that escitalopram would increase amygdala and decrease IFG BOLD responses during the processing of high-arousal positive images and that escitalopram would decrease amygdala and increase IFG BOLD responses during the processing of high-arousal negative images. Based on the expectation that treatment facilitates a positive information processing bias, we also hypothesized that, similar to its effect during the processing of positive stimuli, escitalopram would decrease IFG BOLD responses during the processing of low-arousal neutral images.
Methods
Participants
We recruited right-handed, healthy women by advertisement for participation in our study. We screened the women over the telephone to ensure participants were medication-free (other than hormonal contraceptives) and that they did not have physical and psychiatric illness, symptoms of depression or anxiety, which we assessed using the Patient Health Questionnaire50 and the Generalized Anxiety Disorder 7-item scale.51 Additional exclusion criteria were history of previous psychiatric illness or psychiatric medication use, illicit drug use, alcoholism, smoking, brain injury, neurologic disorders, loss of consciousness for longer than 5 minutes and contraindications for fMRI scanning. Finally, we asked participants to abstain from caffeine on the morning of the experiment. All participants provided informed consent in accordance with the Australian National Health and Medical Research Council (NHMRC) guidelines. The Sydney University Human Research Ethics Committee (13901), and the Northern Sydney Central Coast Area Health Service Human Research Ethics Committee (1105–178M) granted ethical approval. This trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR; ACTRN12611000719932).
Experimental and emotion processing task design
All participants were tested under placebo (saccharin) and escitalopram (20 mg administered orally) conditions using a randomized, double-blind, placebo-controlled crossover design with a washout period of 1 week (or 5 half-lives; t1/2 = 26.7 h52). An equal number of participants was assigned to receive either treatment or placebo in their first testing session. Functional MRI during an emotion processing task was conducted 4 hours post-treatment to coincide with expected peak pharmacokinetic effects of escitalopram (mean tmax = 4.0 h;53 tmax = 3.0 ± 1.5 h52). A crossover design and checks for correct experimental manipulation (instead of a mixed-models approach) were used, as previously recommended and discussed.54,55
A block design emotion processing task was constructed with 3 blocks of high-arousal positive pictures, 3 blocks of negative valance pictures, 3 blocks of low-arousal neutral valance pictures and 9 blocks of fixation crosses (see the Appendix, available at jpn.ca). Stimuli were selected from IAPS18 to elicit complex emotion processing.56 The blocks of fixation crosses were followed by each IAPS picture block. Each of the blocks consisted of 5 pictures presented for 4 seconds each. During all trials pictures were unique within and across sessions, with participants having been randomly assigned to either of 2 forms of the task in their first session. Blocks of pictures were randomized within each form. A number of hormonal, behavioural and neurophysiological manipulation checks were performed (see the Appendix). These manipulation checks included assessment of impact of potential unblinding of treatment owing to the subjective occurrence of side effects on the results of the study. The assessments showed no impact on the results (see the Appendix).
Functional MRI data acquisition
Imaging was performed at Advanced Research and Clinical Highfield Imaging, a dedicated research facility, using a 3.0 T Siemens Trio scanner. Thirty-six consecutive axial slices (4 mm thickness) parallel to the anterior–posterior commissure covering the whole brain were imaged using a T2*-weighted gradient-echo echo-planar imaging sequence (echo time [TE] 32 ms, repetition time [TR] 2000 ms, matrix 64 × 64, flip angle 90°). The field of view was 240 mm and the effective in-plane functional spatial resolution was 3.75 mm. For each functional run, 360 volumes were collected after discarding the first 6. For anatomic reference, high-resolution whole brain images were also acquired under the following parameters: TR 1570 ms, TE 3.22 ms, flip angle 15°, matrix 512 × 512 × 192 mm. Participant movement was minimized by securing the head within the coil using padding.
Functional MRI data analyses
The imaging data were preprocessed and analyzed using the image processing routines implemented within the statistical parametric mapping software package SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8/; Wellcome Trust Centre for Neuroimaging). Images for each participant were first corrected for susceptibility-by-movement artifacts and then realigned to the first volume of the time series. Realigned images were spatially normalized into standard stereotactic space (Montreal Neurological Institute [MNI] template) and smoothed with an 8 mm full-width at half-maximum Gaussian kernel to minimize anatomic differences. The BOLD response at each voxel was modelled with a canonical hemodynamic response function and its temporal derivative.
For each participant, brain activation contrast images were determined from the positive, negative and neutral images contrasted against fixation images at each treatment session. These individual contrast images were then used in the second-level random-effects model to determine regional responses for the whole sample. We conducted a whole brain analysis to ensure the emotion processing task and treatments activated regions associated with affective processing and with our ROIs, which were the left and right amygdala and the left and right IFG (as previously defined57,58). For the whole brain analysis, we used an α threshold of p < 0.05, family-wise error (FWE)–corrected. For all a priori ROI analyses, we used an α threshold of p < 0.05 (partial volume, uncorrected) and a spatial extent of 20 or more voxels per cluster to control for false-discovery rates associated with multiple comparisons within the ROIs, in accordance with accepted guidelines.59,60 To determine the interaction effect of treatment (escitalopram, placebo) × valence (positive, negative, neutral), we performed an analysis of variance (ANOVA) on the valance > fixation contrasts for the amygdala and IFG ROIs. This analysis was then followed up with dependent samples t tests to determine the direction of the effects. For each participant at each session, percent signal changes for each of the positive, negative and neutral pictures were estimated using MarsBaR.61 The ROIs for percent signal change analysis were determined from the significant SPM amygdala and IFG clusters at the second-level analysis. This analysis was conducted to determine the effect of treatment on emotion processing.
Results
Participants
We enrolled 36 women (mean age 25.08 ± 6.49 yr) who met our study inclusion criteria. Years of education ranged from 13 (completed high school) to 23 (completed graduate school). All participants were of white European ethnogeographic ancestry. No participant tested positive on pregnancy tests conducted at each session.
Functional MRI results: whole brain and ROIs
The whole brain analysis of the average effect of picture presentation collapsing across treatment conditions showed significant activation of the left amygdala and bilateral IFG at p < 0.05, FWE-corrected (for all other activations, see the Appendix, Table S1). Therefore, these ROIs were used in consequent analyses. The ROI analysis on the main effect for the emotion processing task revealed significant activations for all ROIs (all p < 0.05, partial volume, false-discovery rate [FDR]–corrected). See the Appendix, Table S2, for statistics for the emotion processing task effects with follow-up tests. The ROI analysis on the main effect of treatment revealed significant activation for the left IFG (k = 59, p = 0.012, uncorrected). See the Appendix, Table S3, for statistics for treatment effects with follow-up tests.
Functional MRI results: treatment × emotion processing interaction
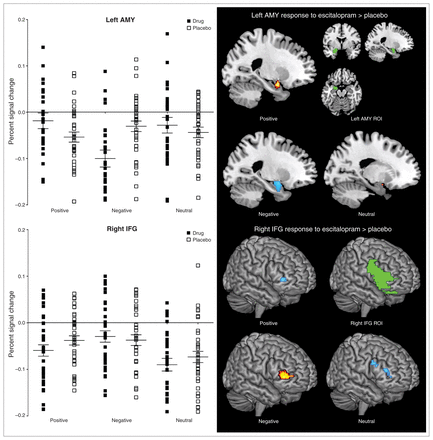
The ROI analysis on the interaction effect of treatment and emotion processing revealed significant activation within the right IFG (k = 283, p < 0.001, uncorrected) and left amygdala (k = 50, p = 0.006, uncorrected). See Table 1 for statistics for the interaction effect between treatment and emotion processing with follow-up tests. The follow-up tests demonstrated that, relative to placebo, escitalopram increased left amygdala and decreased right IFG activation during positive emotion processing; decreased bilateral amygdala activation and increased right IFG activation during negative emotion processing; and decreased right IFG activation during neutral emotion processing. Consistent with these follow-up tests, the percent signal changes obtained at the individual participant level showed increased left amygdala activation during positive stimuli and decreased left amygdala activation in response to negative emotion processing with escitalopram relative to placebo. Increased right IFG activity during negative stimuli and decreased right IFG activation during positive stimuli was shown with escitalopram relative to placebo. Parallel to the positive stimuli, percent signal changes for the neutral stimuli showed decreased right IFG activation with escitalopram relative to placebo (Fig. 1).
Percent signal change and regional activation illustrating a treatment-emotion processing interaction (error bars represent standard error of the mean). AMY = amygdala; IFG = inferior frontal gyrus; ROI = region of interest.
Peak amygdala and IFG activation of the interaction between treatment and affective experience
Discussion
The present study examined the impact of a single dose of the SSRI escitalopram on the processing of high-arousal positive and negative pictures and low-arousal neutral pictures in healthy participants using pharmaco-fMRI. Building on previous findings,10,31 we found that a single dose of escitalopram increased amygdala activation and decreased IFG activation during the processing of positive images. By contrast, escitalopram decreased amygdala activation and increased IFG activation during the processing of negative images, consistent with previous findings. During the processing of low-arousal neutral images, escitalopram decreased IFG activation paralleling the decrease in IFG activation during the processing of positive stimuli. These findings suggest a valence-specific modulation of emotion processing regions with a single dose of escitalopram.
Consistent with predictions based on our earlier EEG findings,10 the present pharmaco-fMRI study found that escitalopram potentiated positive but attenuated negative emotion processing. This was demonstrated by an increase in amygdala activation (which may reflect increased salience21,22) and a decrease in IFG activation (which may reflect reduced reappraisal and inhibition23–25) during the processing of positive pictures and a decrease in amygdala activation (associated with decreased salience) and an increase in IFG activation (reappraisal and greater inhibition) during the processing of negative pictures. The present study’s findings with escitalopram and negative pictures are consistent with those of previous pharmaco-fMRI studies that found decreased amygdala activation in response to negative pictures after a single dose of fluvox-amine31 and citalopram40 and negative faces after a single dose11 or a 7- to 10-day38 treatment of citalopram. Furthermore, the increase in amygdala activation in response to positive pictures with a single dose of escitalopram is consistent with findings from studies on the effect of positive faces following 7- to 10-day treatment with citalopram.38 To our knowledge, the present study is the first to demonstrate increased amygdala activation in response to positive pictures after a single dose of an SSRI.
With respect to neutral pictures, escitalopram decreased IFG activity (reduced reappraisal and inhibition) in a similar manner to that observed during the processing of positive pictures. This observation is novel and suggests that a single dose of a commonly prescribed antidepressant may begin to facilitate a positive bias or decrease negative bias to neutral pictures by reducing negative reappraisal. While a previous investigation observed no change with neutral faces following treatment,11 our findings may be explained by increased complexity and associated increases in frontal engagement associated with appraisal processes of images that is subsequently impacted by treatment.42,43 Our observations that a single dose of escitalopram reduced IFG activity associated with appraisal and inhibition, akin to the positive stimuli, is important given that neutral stimuli tend to be experienced negatively by clinical samples7–9 and that chronic treatment is associated with viewing these stimuli more positively.8 This finding also has implications for pharmaco-fMRI methodology in that employing neutral stimuli as a contrast against emotional stimuli may be problematic as treatment appears to impact on emotional as well as neutral stimulus categories. Future work should determine whether the processing of neutral stimuli becomes more positive or less negative, starting from a single dose.
In accordance with cognitive neuropsychological models of antidepressant action,3,4 the acute neurophysiological changes of escitalopram during presentation of positive, negative and neutral images were not paralleled by changes in subjective ratings of the valence of the stimuli (see the Appendix). Models suggest that with chronic antidepressant treatment changes in affect come with neuroadaptive changes and environmental exposure that further potentiate and consolidate positive emotion and information processing bias.3,4 To test these models and determine whether neural changes increase over time, future research should examine these measurable changes, tracking them over the first dose, the acute/early period and later time periods of therapeutic treatment. Further support for these models would stem from examination of graduated dose–response changes in emotion processing biases with graduated doses of antidepressant treatment. Critically, future research is needed to examine whether the measureable, small to moderate neural changes with single dose antidepressant administration on positive, negative and neutral stimuli processing are predictive of longitudinal changes in affect with chronic treatment in patients with affective disorders. A single dose rather than a subchronic or chronic experimental medicine model may benefit future research on the prediction of therapeutic response in patients and for treatment development both in terms of time and cost.
The present findings suggest that the acute changes in emotion bias characterized in cognitive neuropsychological models2–4 may be further illustrated, at least in part, by anti-depressants’ potentiation of positive emotion and attenuation of negative emotion, with potentiation being associated with a profile of increased amygdala and decreased IFG activation, and attenuation being associated with the reverse profile (decreased amygdala and increased IFG activation). These findings are consistent with an increasing positive bias. In addition, a potential increase in positive bias or decrease in negative bias toward neutral stimuli (decreased IFG activation paralleling responses with positive stimuli) may be a novel characterization of the acute changes with antidepressant treatment. These considerations have been integrated into a cognitive neuropsychological model of antidepressant action3 displayed in the Appendix, Figure S2. Future neuroimaging studies involving emotion regulation tasks may help to further illustrate interactions between these regions.
The acute effects of antidepressants appear to be similar between healthy11,30,31 and clinical samples,32,33,62 with studies reporting increased frontal and decreased limbic activation in response to negatively valenced stimuli and decreased frontal and increased limbic activation in response to positively valenced stimuli. This suggests that single dose outcomes in healthy individuals would be expected to have parallels to those in patients. Circumventing the potential for the moderating effects of illness, treatment, sex, race and associated factors, the present study used a homogeneous, well-characterized sample of healthy, white, European women. Nevertheless, the findings of the present study, particularly the novel findings pertaining to treatment modulation of neutral stimuli, need to be replicated in clinical samples. While neurophysiological measures of the acute effects of antidepressants hold promise for the prediction of their clinical efficacy,5,34 the predictive ability of cognitive neuropsychological models of antidepressant action remains to be tested.63
Limitations
A potential limitation of the present study was the potential un-blinding of treatment condition (see the Appendix), with participants correctly guessing treatment condition over and above chance. This guessing was associated with the subjective experience of side effects, consistent with those typically experienced with escitalopram.47 However, guessing or side effects did not have effects on BOLD activation of the amygdala or IFG during the emotion processing conditions (see the Appendix). Therefore, it is unlikely that unblinding and side effects confounded the results of our study. Nonetheless, future research may consider using an active placebo condition to control for the potential impact of side effects on treatment and emotion processing. Another possible limitation of the present study was that we used a women-only sample. Though we did not find differential impacts of hormonal or menstrual status on BOLD activation during emotion processing under each treatment condition (see the Appendix), our findings may not be generalizable to men. As men have different patterns of neurophysiological responses to affective stimuli64 that are further differentiated by the valence of the stimuli,65 it is imperative to extend our findings to men in future studies, as drug effects may differ. Nevertheless, the within-subjects design and extensive manipulation checks, along with the use of a homogeneous, well-characterized sample, increase our confidence in the observed findings.
Conclusion
To our knowledge, we demonstrate for the first time that a commonly prescribed SSRI antidepressant, escitalopram, differentially impacts neural activation consistent with an increasing positive bias toward positive and against negative emotional stimuli. In addition, we observed a reduction in the appraisal of neutral stimuli, which may have implications for depressed individuals, who appraise such stimuli negatively. Our study also has important methodological implications with regards to baseline conditions in studies on the effects of antidepressants, as treatment may impact on neutral as well as emotional stimulus conditions. In addition to providing important new data on the more commonly prescribed anti-depressant escitalopram, our novel findings support and extend cognitive neuropsychological models of antidepressant action. Further work in this domain may enable the development of acute measures to predict treatment efficacy.
Acknowledgements
This research was supported by an Australian Research Council Discovery Project Grant (DP0987332), a National Health and Medical Research Council (NHMRC) Project Grant (464863) and a NHMRC Career Development Award (571101) awarded to A.H. Kemp. We acknowledge the support and assistance of the local and international research students and laboratory volunteers throughout the project.
Footnotes
Competing interests: T. Outhred and A.H. Kemp are supported by an Australian Postgraduate Award and an International Research Professorship from the Universidade de São Paulo, respectively. P.J. Nathan is an employee at UCB Pharma and hold shares in the company. G.S. Malhi has received research support from AstraZeneca, Eli Lilly, Organon, Pfizer, Servier and Wyeth. He has been a speaker for Astra-Zeneca, Eli Lilly, Janssen Cilag, Lundbeck, Pfizer, Ranbaxy, Servier and Wyeth. He has been a consultant for AstraZeneca, Eli Lilly, Janssen Cilag, Lundbeck and Servier. None declared for P. Das, K.L. Felmingham and R.A. Bryant.
Contributors: A.H. Kemp acquired the funding to conduct the study. T. Outhred, P. Das, K.L. Felmingham, R.A. Bryant, G.S. Malhi and A.H. Kemp designed the study. T. Outhred, P. Das, G.S. Malhi and A.H. Kemp acquired and analyzed the data, which P.J. Nathan also analyzed. T. Outhred wrote the article, which all authors reviewed and approved for publication.
- Received June 18, 2013.
- Revision received November 3, 2013.
- Revision received January 7, 2014.
- Accepted January 14, 2014.