Abstract
Background: Metabotropic glutamate receptors 2/3 (mGluR2/3) and 5 (mGluR5) are novel therapeutic targets for major depression (MD), bipolar disorder (BD) and schizophrenia. We aimed to determine whether mGluR2/3 and mGluR5 binding in the anterior cingulate cortex (ACC), a brain region essential for the regulation of mood, cognition and emotion, were differentially altered in these pathologies.
Methods: Using postmortem human brains derived from 2 cohorts, [3H]LY341495 binding to mGluR2/3 and [3H]MPEP binding to mGluR5 were measured by receptor autoradiography in the ACC. The first cohort comprised samples from individuals who had MD with psychosis (MDP), MD without psychosis (MDNP) and matched controls (n = 11–12 per group). The second cohort comprised samples from individuals who had MDNP, BD, schizophrenia and matched controls (n = 15 per group).
Results: No differences in mGluR2/3 or mGluR5 binding were observed in the MDP, MDNP, BD or schizophrenia groups compared with the control group (all p > 0.05). Importantly, there were also no differences in binding densities between the psychiatric disorders (p > 0.05). We did, however, observe age-related effects, with consistent negative associations between mGluR2/3 and age in the control group (r < −0.575, p < 0.025) and the psychotic disorder groups (MDP and schizophrenia: r = −0.765 to −0.515, p < 0.05), but not in the mood disorder groups (MDNP, BD).
Limitations: Replication in larger independent cohorts and medication-naive individuals would strengthen these findings.
Conclusion: Our findings suggest that mGluRs are unaltered in the ACC; however, the presence of altered receptor function cannot be discounted and requires further investigation. Taken together with previous studies, which report differential changes in mGluR2, 3 and 5 across these disorders, we suggest mGluRs may be affected in a brain region–specific manner.
Introduction
Major depression (MD) is an important cause of disability as well as financial and emotional burden worldwide. While traditionally the monoaminergic system has been implicated in the pathophysiology and treatment of MD, evidence from clinical, preclinical and postmortem studies are rapidly implicating glutamatergic dysregulation in MD.1 Accordingly, metabotropic glutamate receptors (mGluRs), which slowly mediate glutamatergic neurotransmission, are promising therapeutic targets for the treatment of MD. The challenges, however, in elucidating the pathophysiology of MD, and therefore appropriate treatment strategies, are the heterogeneity of the disorder and its association with other psychiatric and mood disorders, such as bipolar disorder (BD) and schizophrenia.
Major depression with psychotic features (psychotic depression) is classified as a subtype of major depression in DSM-IV. As recently discussed by Rothschild,2 psychotic depression is associated with BD. Studies have shown that patients with psychotic depression had an increased risk for BD compared with patients with nonpsychotic depression. In contrast, evidence suggests psychotic depression is distinct from schizophrenia.2 It is necessary to characterize the pathophysiology of depression, including its psychotic and nonpsychotic types, and determine the differences and similarities between BD and schizophrenia, as this has crucial implications for advising the use of certain pharmacotherapeutics.
Several lines of evidence suggest the involvement of the anterior cingulate cortex (ACC) in the pathophysiology of depression. It has critical control over mood, emotion and cognition, as proven by bilateral cingulotomy psychosurgery, which can relieve depression and some psychiatric symptoms.3 Concurrently, reduced glutamate levels have been reported in the ACC of patients with depression,4–6 and this is reflected at the protein level for various markers expressed at the glutamatergic synapse.7–9 The involvement of glutamatergic dysfunction in depression may be specific to this brain region, as 1 group found alterations of various postsynaptic glutamatergic proteins (NR1 subunit of the N-methyl-d-aspartate receptor [NMDAR], postsynaptic density protein 95 [PSD95] and postsynaptic density protein 93 [PSD93]) in the ACC, but not in the dorsolateral prefrontal cortex (DLPFC), in individuals with depression.7
Metabotropic glutamate receptors, particularly mGluR2/3 and mGluR5, have been identified as novel therapeutic targets for mood and psychiatric disorders because of their ability to modulate glutamatergic neurotransmission.10–12 Whereas mGluR2/3 are predominately presynaptic receptors that inhibit glutamate and γ-aminobutyric acid (GABA) release into the synapse,13 mGluR5 are primarily expressed postsynaptically, where they modulate the glutamate response, especially in association with the NMDAR.11 In addition to their therapeutic potential, mGluRs are also implicated in the pathophysiology of these disorders. For example, numerous animal studies demonstrate that selected mGluRs are affected in animal models of depression,14,15 and knockout animals display psychotic phenotypic behaviours.11,13 From these studies, it is becoming increasingly clear that there may be opposing actions of the glutamatergic system in these disorders, particularly as mGluR2/3 and mGluR5 blockade appear to be beneficial for the treatment of depression, while potentiation of these receptors has preclinical efficacy for the treatment of psychosis.1,11,13 Hence, mGluR2/3 and mGluR5 may be differentially involved in the pathophysiology of mood and psychotic disorders.
We sought to determine whether mGluR2/3 and mGluR5 were altered in the ACC of individuals with MD (in the presence and absence of psychosis) and the associated pathologies BD and schizophrenia. We studied 2 independent postmortem cohorts from the Stanley Medical Research Institute: the Stanley Depression Collection, consisting of samples from individuals who had MD without psychosis (MDNP), MD with psychosis (MDP) and controls, and the Stanley Neuropathology Consortium, consisting of samples from individuals who had diagnoses of MDNP, BD and schizophrenia and controls. We measured mGluR2/3 and mGluR5 binding in the ACC (Brodmann area [BA] 24) of these samples by in situ receptor autoradiography. As the ACC is implicated in the emotional and cognitive deficits associated with these disorders, the findings from this study have translational implications for novel drugs aimed to treat mood and cognitive deficits in patients with these disorders.
Methods
Human postmortem brain samples
Frozen postmortem brain tissue was acquired through the Stanley Medical Research Institute. From the Depression Collection, we obtained samples from 24 individuals who had MD (MDNP: n = 12; MDP: n = 11) and controls (n = 12) matched according to age, postmortem interval (PMI), refrigeration interval and brain pH (Table 1). Psychosis could not be confirmed in 1 of the MD samples, who was therefore excluded from psychosis-specific analyses. From the Neuropathology Consortium, we obtained samples from 60 individuals who had MDNP, BD and schizophrenia and controls (n = 15 per group), matched for age, sex, race and PMI (Table 2). Detailed clinical and demographic information regarding these cohorts have been published previously.16,17 The work described in this study was approved by the Human Research Ethics Committee at the University of Wollongong and conducted according to their guidelines (HE13/069).
Cohort demographic and clinical descriptives of tissue sample donors within the Depression Collection
Cohort demographic and clinical descriptives of tissue sample donors within the Neuropathology Consortium
Receptor autoradiography
Frozen coronal sections at the level of the ACC (BA24; 14 μm) were mounted onto slides and stored at −80°C until the day of the assay. Receptor autoradiography studies were based on protocols described previously (mGluR2/3;18,19 mGluR520,21). Autoradiography exposures were optimized for mGluR2/3 and mGluR5 to ensure that each cortical layer could be quantified on a linear scale and none of the cortical layers with high expression were overexposed. The signal to noise ratio of mGluR2/3 and mGluR5 binding in all groups assayed were well above the nonspecific control. Two sections for each individual were analyzed for total binding, and adjacent sections were used to determine nonspecific binding. All experiments were performed blind to diagnoses.
mGluR2/3
Tissue sections were preincubated in a buffer solution containing 50 mM Tris buffer, 2 mM MgCl2 and 2 mM CaCl2 (pH 7) at room temperature for 2 × 10 minutes. Subsequently, slides were incubated for 60 minutes at room temperature in buffer solution containing 50 nM [3H]LY341495 (specific mGluR2/3 agonist; specific activity 40 Ci/mmol; American Radiolabelled Chemicals). To determine nonspecific binding, adjacent sections were additionally incubated with 50 nM [3H]LY341495 and 0.01 mM DCG-IV (specific mGluR2/3 agonist; Sigma). After incubation, the slides were washed in 4°C washing buffer (50 mM Tris buffer, pH 7) for 2 × 30 seconds and 1 × 1 minute. This protocol favours mGluR2 over mGluR3 binding.19
mGluR5
Tissue sections were preincubated for 15 minutes at room temperature in 50 mM TrisNaCl (pH 7.5). Slides were then incubated in solution containing 10 nM [3H]MPEP (specific mGluR5 antagonist; specific activity 60 Ci/mmol; American Radiolabelled Chemicals) and 50 mM TrisNaCl for 90 minutes at room temperature. Adjacent sections were incubated in the same solution with the addition of 10 μM unlabelled MPEP (Sigma) to determine nonspecific binding. Slides were washed in 4°C TrisNaCl (pH 7.5) for 2 × 3 minutes and briefly dipped in 4°C MilliQ-water.
Imaging and quantification
Together with [3H] microscale autoradiographic standards (Amersham Biosciences), sections were exposed to tritium sensitive Kodak Biomax MR film (Kodak) for 10 weeks. Films were developed using the AGFA CP1000 film developer (Agfa-Gavaert N.V.), scanned using a GS800 densitometer (BioRad) and analyzed using Multi-Analyst software (Bio-Rad). Quantification was performed blind to diagnoses. Radioligand binding signals were expressed in counts per minute (cpm)/mm2 and, with the use of standards and the specific activities of the respective radioligands, were converted to fmol/mg tissue equivalents.
Statistical analysis
As data were normally distributed across both cohorts, we used parametric tests. No data points were identified as outliers (± 2 standard deviations). The depression collection and the neuropathology consortium were analyzed separately. We used analysis of variance (ANOVA) followed by a Tukey post hoc test to compare differences in mGluR2/3 and mGluR5 binding between the diagnoses in the 2 cohorts (i.e., MDNP, MDP v. control and MDNP, BD, schizophrenia and control). Subsequently, we completed Pearson correlations to determine any influence of continuous variables on the data. We then carried out analyses of covariance (ANCOVAs) in which we compared binding values between the diagnostic groups in the 2 cohorts, controlling for variables that significantly correlated with the data. Statistical analyses were performed using SPSS software version 19.0. We considered results to be significant at p < 0.05, and data are presented as means ± standard errors of the mean (SEM).
The Stanley Neuropathology Consortium Integrative Database
The Stanley Neuropathology Consortium Integrative Database (SNCID; http://sncid.stanleyresearch.org)22 includes 1762 pathological markers measured in 12 different brain regions by the Neuropathology Consortium. In line with the hypotheses of the present study, we sought to determine if there were associations between our measures of mGluR2/3 and mGluR5 and measures of other glutamate-related proteins, specifically within the cingulate cortex. Exploratory correlation analyses of glutamatergic markers (various measures of NMDAR, 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl propionate receptor [AMPAR] and kainate receptors) and signalling partners (PSD95, brain-derived neurotrophic factor [BDNF] and protein kinase C [PKC]) that have previously been measured in the cingulate cortex (BA not specified) was performed using the SNCID as previously described.22 We used a nonparametric method (Spearman correlation) to minimize effects caused by differences in units and/or distribution patterns.23 All 60 individuals were included, and significance was set at p < 0.05. Detailed information regarding the measurement and experimental protocol for each marker is available on the SNCID database (http://sncid.stanleyresearch.org).
Results
Distribution
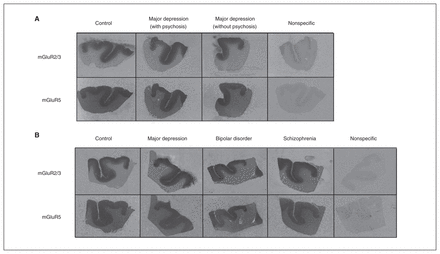
We found that mGluR2/3 ([3H]LY341495) and mGluR5 ([3H] MPEP) binding were highly expressed in the cortical areas of brain slices across the 2 cohorts (Fig. 1). Whereas mGluR5 binding was homogeneously distributed across all cortical layers, mGluR2/3 binding showed pronounced layer differences; the superficial one-third of the cortex had significantly higher binding than the lower layers (p < 0.001) and was analyzed accordingly.
Representative receptor autoradiographs of mGluR2/3 [3H]LY341495 and mGluR5 [3H]MPEP binding from the anterior cingulate cortex of samples from the (A) Stanley Depression Collection (control, major depression with psychosis, major depression without psychosis) and the (B) Stanley Neuropathology Consortium (control, major depression, bipolar disorder and schizophrenia). A representative nonspecific binding has been included for each cohort. mGluR2/3 [3H]LY341495 and mGluR5 [3H]MPEP binding densities were not different between pathological and control groups.
Depression Collection
Analysis of demographic and clinical data
Mean pH, age at death, PMI and brain weight did not differ among the MDNP, MDP and control groups (F = 0.054–3.171, all p > 0.05). Excluding controls, further analyses revealed no significant differences between diagnoses (MDNP v. MDP) for age of onset (t21 = −0.379, p = 0.71) or illness duration (t21 = −0.417, p = 0.68). Individuals in the MDNP and MDP groups were exposed to antipsychotic medication (measured as fluphenazine equivalent units), although average estimated lifetime exposure did not differ significantly between the groups (3 in the MDNP group v. 9 in the MDP group, t10 = 0.940, p = 0.37). Quantitative antidepressant and mood stabilizer medication histories were not available.
Diagnosis-related effects
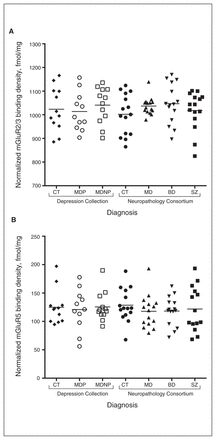
One-way ANOVA revealed no significant diagnostic alterations in mGluR2/3 (total: F2,32 = 0.306, p = 0.74; upper: F2,32 = 1.502, p = 0.24; lower: F2,32 = 0.338, p = 0.72) or mGluR5 (F2,32 = 0.074, p = 0.93) comparing the MDP, MDNP and control groups (Fig. 2). Subsequently, we performed ANCOVAs to control for age at death, brain pH, refrigeration interval, brain weight and PMI; however, significance was unchanged for mGluR2/3 (total: F2,27 = 0.934, p = 0.43; upper: F2,27 = 0.761, p = 0.72; lower: F2,27 = 0.025, p = 0.98) or mGluR5 (F2,27 = 1.010, p = 0.46). In addition, we performed an independent t test to compare all individuals with depression (n = 24) and controls (n = 12). No significance was seen for either mGluR2/3 (total: t34 = −0.009, p = 0.99; upper: t34 = −0.576, p = 0.57; lower: t34 = 0.693, p = 0.49) or mGluR5 (t34 = −0.106, p = 0.92).
Normalized (A) mGluR2/3 [3H]LY341495 and (B) mGluR5 [3H]MPEP binding density (as measured in fmol/mg tissue) in the anterior cingulate cortex of control (CT; diamonds = Depression Collection; solid circles = Neuropathology Consortium), major depression with psychosis (MDP; hollow circles), major depression without psychosis (MDNP; Depression Collection = hollow squares; Neuropathology Consortium = upward arrowheads), bipolar disorder (BD; downward arrowheads), and schizophrenia (SZ; solid squares) groups. mGluR2/3 and mGluR5 binding densities were not altered or differentially expressed in the anterior cingulate cortex in these neuropathologies.
Effects of demographic and clinical variables
Pearson correlations for continuous variables (pH, age at death, PMI, refrigeration interval, brain weight, age at disease onset, duration of illness and lifetime fluphenazine equivalents) are presented in Table 3 and in the Appendix, Table S1, available at jpn.ca. Age at death was consistently associated with mGluR2/3 binding in the control group (total: r = −0.551, p = 0.06; upper: r = −0.747, p = 0.005; lower: r = −0.134; p = 0.94) and in the MDP group (total: r = −0.765, p = 0.006; upper: r = −0.748, p = 0.008; lower: r = −0.692, p = 0.018) but not in the MDNP group (total: r = −0.371, p = −0.24; upper: r = −0.383, p = 0.22; lower: r = −0.335, p = 0.29). Age at death correlated positively with mGluR5 in the MDNP group (r = 0.587, p = 0.045) but not in the MDP group (r = 0.551, p = 0.08) or the control group (r = 0.023, p = 0.94; Table 3).
Pearson correlations for variables influencing mGluR2/3 and mGluR5 binding densities in the anterior cingulate cortex within diagnostic groups of the Depression Collection
Overall, there was no effect of lifetime antipsychotic exposure on total or lower mGluR2/3 (total: r = −0.289, p = 0.19; lower: r = −0.047, p = 0.84) or mGluR5 (r = 0.005, p = 0.98) in all individuals who had MD (MDNP and MDP), but we observed a negative association with mGluR2/3, specifically in the upper layer (r = −0.460, p = 0.031). Power for the subgroups was notably low (MDNP: n = 3; MDP: n = 9) and was therefore not reported (Table 3).
Neuropathology Consortium
Analysis of demographic and clinical data
Mean pH, age at death, PMI and brain weight did not differ among the MDNP, BD, schizophrenia and control groups (F = 0.420–1.857, all p > 0.147). However, freezer storage time was significantly greater in the BD group (281.73 d longer, p = 0.010) and the schizophrenia group (282.86 d longer, p = 0.009) than the control group. Excluding controls, further ANOVAs and post hoc tests revealed significant differences between diagnoses, with the MDNP group having a mean age at onset that was 10.73 years older than the schizophrenia group (p = 0.016) and 12.46 years older than the BD group (p = 0.005). Illness duration also approached significance, being 8.63 years longer in the schizophrenia group than in the MDNP group (p = 0.08). Of the patients who had been exposed to antipsychotic medication (12 in the BD group and 14 in the schizophrenia group), lifetime antipsychotic exposure did not differ between the groups (t24 = −1.557, p = 0.13). Quantitative antidepressant and mood stabilizer medication histories were not available.
Diagnosis-related effects
One-way ANOVA revealed no significant diagnostic alterations in mGluR2/3 (overall: F3,56 = 1.387, p = 0.26; upper: F3,56 = 1.586, p = 0.20; lower: F3,56 = 0.847, p = 0.47) or mGluR5 (F3,56 = 0.541, p = 0.66) binding densities in the MDNP, BD and schizophrenia groups compared with the control group (Fig. 2). The ANCOVA controlling for age at death, brain pH, brain weight, freezer storage time and PMI confirmed this finding for both mGluR2/3 (total: F3,51 = 0.934, p = 0.43; upper: F3,51 = 0.761, p = 0.52; lower: F3,51 = 0.818, p = 0.49 and mGluR5 (F3,51 = 1.010, p = 0.40). As age at disease onset, illness duration and lifetime fluphenazine equivalents were observed to correlate with binding (Table 4 and Appendix, Table S1), we performed additional ANCOVAs excluding control samples. The data remained nonsignificant for both mGluR2/3 (total: F2,35 = 0.998; p = 0.38; upper: F2,35 = 1.203; p = 0.31; lower: F2,35 = 0.497, p = 0.61) and mGluR5 (F2,35 = 0.382, p = 0.69), suggesting no significant difference in mGluR2/3 and mGluR5 binding among the MDNP, BD and schizophrenia groups.
Pearson correlations for variables influencing mGluR2/3 and mGluR5 binding densities in the anterior cingulate cortex within diagnostic groups of the Neuropathology Consortium.
Effects of demographic and clinical variables
Pearson correlations for mGluR2/3 and mGluR5 with continuous variables (pH, age at death, PMI, freezer storage time, brain weight, age at disease onset, duration of illness, and lifetime fluphenazine equivalents) are presented in Table 4 and in the Appendix, Table S1. Various correlations for pH, brain weight, PMI and freezer storage time were observed across the diagnostic groups; these were accounted for by ANCOVA and did not affect the diagnostic findings.
Consistent with our results in the Depression Collection, age at death had a significant negative effect on mGluR2/3 binding in the control group (total: r = −0.695, p = 0.004; upper: r = −0.666, p = 0.007; lower: r = −0.575, p = 0.025) and in the schizophrenia group (total: r = −0.528, p = 0.043; upper: r = −0.458, p = 0.09; lower: r = −0.515, p = 0.049) groups, but not in the MDNP or BD groups. Age at death displayed a borderline significant correlation with mGluR5 in the schizophrenia group (r = 0.505, p = 0.06) but not in the MDNP, BD or control groups. Illness duration also significantly and negatively correlated in the schizophrenia group for mGluR2/3 (total: r = −0.697, p = 0.004; upper: r = −0.734, p = 0.002; lower r = −0.539, p = 0.038), although this correlation was not seen with mGluR5. Although lifetime antipsychotic medication did not differ between the schizophrenia and BD diagnostic groups, a significant negative correlation with mGluR2/3 was observed, specifically in the BD group (Table 4).
Correlations with glutamatergic markers and signalling partners in the SNCID
Explorative Spearman correlations with the data from the present study and those from previous studies (available in the SNCID) are presented in the Appendix, Table S2. Various significant associations were observed, both overall and in individual pathologies, between mGluR2/3 and mGluR5 with measures of NMDAR, AMPAR and kainate receptors as well as signalling partners PSD95, PKC and BDNF.
Discussion
The mGluR2/3 and mGluR5 receptors are targets of new therapeutic approaches for psychiatric conditions, including MD, BD and schizophrenia. Furthermore, animal studies have suggested the involvement of these receptors in the pathophysiologies of these disorders.1,11 In light of this, we have examined binding to mGluR2/3 and mGluR5 in postmortem brains, specifically the ACC, of individuals with MD (MDP, MDNP), BD and schizophrenia and compared them to matched controls. In particular, we chose the receptor binding technique and radioligands that target the same binding sites as novel drugs currently under development to determine how drugs targeting these receptor sites may be affected in the patient. We report no significant diagnostic alterations of mGluR2/3 binding or mGluR5 binding in the ACC in individuals with these pathologies.
Unaltered levels of mGluR2/3 and mGluR5 in depression (with or without psychosis)
mGluR2/3 and mGluR5 are prominent prospective targets in the treatment of mood disorders, as both clinical and pre-clinical studies have shown antidepressant properties of antagonists or negative allosteric modulators targeted at mGluR2/3 and mGluR5.1,12 To our knowledge, we are the first to report that neither mGluR2/3 nor mGluR5 binding levels are altered in the ACC of individuals with MD, in the presence (MDP) or absence (MDNP) of psychosis. Our results are in line with a previous study reporting unaltered mGluR2 and mGluR3 gene expression in the postmortem ACC of individuals with MD.9 We found no other studies that have investigated mGluRs in the ACC in individuals with depression. In other brain regions of depressed patients, an increase in mGluR2/324 and reduction of mGluR5 protein in the prefrontal cortex (BA10) have been reported, together with reduced (in vivo) prefrontal cortical mGluR5 binding25 and increased mGluR5 gene expression in the locus coeruleus.26 Although it is difficult to draw conclusions from only a small number of studies, the inconsistencies between these findings highlight the inherent regional differences in mGluRs in the pathophysiology of MD as well as the importance of considering psychiatric subclass. These prior studies have an important limitation in that they do not distinguish between MDP and MDNP, particularly since differential proteome profiles have been recently reported, specifically between MDNP and MDP.17
mGluR2/3 and mGluR5 binding does not differ in BD and schizophrenia compared with depression
In line with depression with and without psychosis we report that mGluR2/3 and mGluR5 binding density are unaltered in the ACC in individuals with schizophrenia and BD. While mGluR binding, to our knowledge, has not previously been measured in individuals with BD, studies in the prefrontal cortex, thalamus and striatum of individuals with schizophrenia have likewise reported no change in mGluR2/319,27–30 or mGluR511,21 (binding, protein or mRNA). Our findings highlight that in the ACC there are no distinct differences between the overlapping diagnoses of MDNP, MDP, schizophrenia and BD with regards to binding to mGluR2/3 and mGluR5. This suggests that novel pharmacological modulators of mGluR2/3 and mGluR5 may have unhindered binding sites in all these disorders.
It should, however, be noted that there may be wider dysregulations in mGluR2/3 and mGluR5 function that were not measured within the scope of our study or that there may be alterations in other mGluR subtypes dependent on specific brain regions.12 For example, Volk and colleagues31 found reductions in mGluR1a mRNA, but not mGluR5, in the prefrontal cortex (BA9 and BA42) of patients with schizophrenia. Conversely another more recent study found mGluR5 mRNA and protein to be reduced specifically in BA9 and the lateral cerebellum in individuals with schizophrenia.32 These examples highlight the heterogeneity and intricacies of spectrum disorders such as schizophrenia.33
Current and novel pharmacotherapy implications
In the present study we found evidence of a negative association between mGluR2/3 binding and antipsychotic dose, specifically in individuals with BD and MD, but not in those with schizophrenia. Previous postmortem studies have reported no association between antipsychotic dose and mGluR2/319,27,28 or mGluR5,21 specifically in patients with schizophrenia. Animal studies have reported no effect of antipsychotics on mGluR2/334 or mGluR5 binding.21 The effects of antidepressants have not been as well studied, and while reliable quantitative lifetime antidepressant drug history was not available in the present study, our previous work found no association of antidepressant history and mGluR5 binding and protein in the DLPFC in individuals with schizophrenia.21 Deschwanden and colleagues,25 however, found that antidepressant treatment may reduce mGluR5 binding specifically in the precentral gyrus of antemortem patients with MD. Animal studies have shown that tricyclic antidepressant treatment significantly increases mGluR2/335 and mGluR5a36 protein in the rat hippocampus, although another study found that total mGluR5 protein levels were unaltered.35 To our knowledge, there are no prior studies on antidepressant effects specifically in the ACC to shed light on these findings; therefore, further investigation is required.
The mGluR-targeted modulators have shown promising antipsychotic and antidepressant therapeutic potential in preclinical and clinical studies. Orthosteric and allosteric modulators of mGluRs target binding sites on either the N-terminus (mGluR2/3) or transmembrane (mGluR5) domain, respectively.1,11,13,37 These orthosteric and allosteric binding sites were the same targets of the radioligands used in the present study. As we report that these binding sites are not altered in the ACC in psychopathology, mGluR2/3 and mGluR5 may be unimpeded targets for novel antidepressant and antipsychotic intervention. As results from recent clinical trials with mGluR modulators indicated that specific single nucleotide polymorphisms (SNPs) were associated with treatment response,38 further study into these same binding sites in individuals with these SNPs would be of value. In addition, we consistently found a negative correlation between mGluR2/3 binding and age in the control, schizophrenia and MDP groups, with no association in the MDNP and BD groups. Age-related differences in treatment approaches remain an issue,39 and our findings suggest there may be differential treatment responses to these novel therapeutics depending on the pathology and age of the patient.
Association of mGluRs with markers in the wider glutamatergic system
In addition to the metabotropic group of glutamate receptors, glutamate signalling is also transduced through the ionotropic glutamate receptors NMDAR, AMPAR and kainate receptors. The NMDAR and AMPAR receptors are of particular interest as they signal closely with mGluR511 and mGluR2/3,12 respectively, and novel mGluR2/3- and 5- based drugs are proposed to exert their therapeutic effects, at least partially, through their influence on NMDAR and AMPAR.11,12 In addition, PSD-95 is a key signalling partner of mGluR5 as it physically and functionally links it to NMDAR in the postsynapse, while PKC and BDNF are downstream signalling effectors of mGluR5/NMDAR signalling.11 We explored the association of mGluR2/3 and mGluR5 measured in the present study in relation to measures of these glutamatergic markers as available on the SNCID. We chose a total of 29 markers representing glutamatergic markers and signalling partners within the cingulate cortex.
As expected, we observed a number of differential associations between mGluR2/3, mGluR5 and these glutamatergic markers and signalling partners (see the Appendix, Table S2). Many of these associations were specific to 1 of the 3 psychiatric disorders examined, indicating that there may be differential associations in the pathophysiologies of these disorders. However, although correlation analyses are useful to identify associations between markers, they do not indicate causality and must be treated as exploratory. These associations may provide direction for future studies aiming to distinguish pathological differences between these psychiatric disorders.
Limitations
While our findings suggest possible age-related differences between the psychotic and mood components, further analysis in a larger postmortem cohort is required to support this notion. In addition, further studies are required to determine if mGluR2/3 or mGluR5 function or downstream signalling are altered in these pathologies. It should be noted that despite no pathological alteration of mGluR2/3 and mGluR5 binding sites in the present study, the ACC remains highly involved in these pathologies as alterations of various neurotransmitters and their associated receptors have been reported in this brain region.3,7,40–42 Although our study provides valuable information on these binding sites in psychiatric pathology, additional studies examining binding specifically to membranous mGluR2/3 and mGluR5 and the affinity of novel mGluR2/3- and mGluR5-targeting drugs for these receptors in psychiatric disorders will provide valuable insight into the effectiveness of these drugs in the patient.33
One confound of postmortem studies in neuropsychiatric disorders, is the presence of comorbid substance abuse, especially alcohol and drug abuse, or at least substantially higher alcohol43 and illicit drug44 intake in patients with psychiatric disorders. While reliable quantitative patient histories were not available in the present study, mGluR1/5 densities in the prefrontal cortex may be affected by alcohol,45 and mGluR2 gene expression was reported to be reduced in the ACC of patients with alcohol addiction.46 Furthermore, there is a growing body of evidence suggesting that mGluRs may be involved in drug and alcohol addiction disorders.47 Therefore, the molecular effects of alcohol and drug use should be considered when interpreting the present and prior/future studies.
Conclusion
To our knowledge, we have assessed for the first time mGluR2/3 and mGluR5 binding capacity in the ACC in individuals with MD with and without psychosis as well as those with BD and schizophrenia. While we provide evidence that age is differentially associated with mGluR2/3 binding in individuals with psychotic and mood disorders, we found no alterations in mGluR2/3 or mGluR5 binding compared with controls or between any studied pathological state. This suggests that mGluR2/3- and mGluR5-based therapeutics used to treat emotional and cognitive deficits may have unhindered binding capacity in these pathological states. Future studies may extend on this work by investigating mGluR protein/mRNA expression and their association with other mGluRs, specifically in the ACC and other brain limbic structures; this would increase our understanding of the role of mGluRs in the neuropathologies of psychiatric disease and elucidate their treatment potential.
Acknowledgements
Postmortem tissue was acquired through the Stanley Medical Research Institute, who we thank for the collecting and preparing of tissue specimens. We thank the tissue donors and their families for consenting to the use of this tissue for research. N. Matosin is supported by Australian Rotary Health in the form of an Ian Scott PhD Scholarship.
Footnotes
Funding: This study was funded through an Illawarra Health and Medical Research Institute grant to K.A. Newell, E. Frank, C. Deng and J. Wong. This work was also supported by the Schizophrenia Research Institute, using infrastructure funding from the New South Wales Ministry of Health.
Competing interests: None declared.
Contributors: K.A. Newell designed the study. N. Matosin and K.A. Newell acquired the data, which all authors analyzed. N. Matosin and K.A. Newell wrote the article, which all authors reviewed and approved for publication.
- Received October 22, 2013.
- Revision received February 22, 2014.
- Accepted March 24, 2014.