Abstract
Background: Psychotic symptoms are core clinical features of schizophrenia. We tested recent hypotheses proposing that psychotic, or positive, symptoms stem from irregularities in long-range white matter tracts projecting into the frontal cortex, and we predicted that selective dopamine D2/3 receptor blockade would restore white matter.
Methods: Between December 2008 and July 2011, antipsychoticnaive patients with first-episode schizophrenia and matched healthy controls underwent baseline examination with 3 T MRI diffusion tensor imaging and clinical assessments. We assessed group differences of fractional anisotropy (FA) using voxelwise tract-based spatial statistics (TBSS) and anatomic region of interest (ROI)–based analyses. Subsequently, patients underwent 6 weeks of antipsychotic monotherapy with amisulpride. We repeated the examinations after 6 weeks.
Results: We included 38 patients with first-episode schizophrenia and 38 controls in our analysis, and 28 individuals in each group completed the study. At baseline, whole brain TBSS analyses revealed lower FA in patients in the right anterior thalamic radiation (ATR), right cingulum, right inferior longitudinal fasciculus and right corticospinal tract (CT). Fractional anisotropy in the right ATR correlated with positive symptoms (z = 2.64, p = 0.008). The ROI analyses showed significant associations between positive symptoms and FA of the frontal fasciculi, specifically the right arcuate fasciculus (z = 2.83, p = 0.005) and right superior longitudinal fasciculus (z = −3.31, p = 0.001). At re-examination, all correlations between positive symptoms and frontal fasciculi had resolved. Fractional anisotropy in the ATR increased more in patients than in controls (z = −4.92, p < 0.001). The amisulpride dose correlated positively with FA changes in the right CT (t = 2.52, p = 0.019).
Limitations: Smoking and a previous diagnosis of substance abuse were potential confounders. Long-term effects of amisulpride on white matter were not evaluated.
Conclusion: Antipsychotic-naive patients with schizophrenia displayed subtle deficits in white matter, and psychotic symptoms appeared specifically associated with frontal fasciculi integrity. Six weeks of amisulpride treatment normalized white matter. Potential re-myelinating effects of dopamine D2/3 receptor antagonism warrant further clarification.
Introduction
Schizophrenia is a progressive brain disease characterized by a spectrum of fundamental changes in thinking and beliefs, many of which collectively are referred to as psychotic, or positive, symptoms. Studies using MRI have characterized volumetric brain changes in grey matter and in cerebrospinal fluid.1,2 Although volumetric changes have added to our understanding of illness progression and long-term outcome,3 the field is still left with an incomplete understanding of the biology underlying the characteristic symptoms accompanying schizophrenia.4 In the past 2 decades, in vivo diffusion tensor imaging (DTI) techniques have supported the concept of schizophrenia as a “misconnection syndrome.”5 The vast majority of studies have been cross-sectional, rendering effects of specific antipsychotic compounds, transmitter systems and disease progression largely unexplored.6,7
Two independent research groups recently proposed more comprehensive models of schizophrenia.8,9 The models imply that disturbances in the reward system10,11 may stem from irregularities in myelination and subsequent delayed “corollary discharges.”8,9 Corollary discharges comprise early efferent information, which is initiated by willed actions. Alterations in white matter tracts will increase or decrease the communication between spatially separate brain regions.8 Phenomenologically, abnormal corollary discharges will render the individual estranged to the origin of his or her actions, thoughts and emotions (“perplexity”). Specifically, long-range white matter projections into the higher-order cortical regions of the frontal lobes may be associated with positive symptoms.6,12
At a cellular level, dysfunctional glutamatergic synapses involved in synaptic plasticity may cause the delayed corollary discharges.13 Several neurotransmitters, such as acetylcholine, serotonin and dopamine, modulate the synapses at the glutamate (N-methyl-d-aspartate [NMDA]) receptors.14 Since most antipsychotic compounds affect the levels of these and other neurotransmitters, the effect of specific transmitter systems on white matter integrity is challenging to disentangle. Preclinical15 and clinical studies16 have reported subtle white matter reductions after antipsychotic exposure. Haloperidol may downregulate genes associated with myelin and oligodendrocyte function.17 Conversely, an increase in myelination after exposure to quetiapine and, to a lesser extent, olanzapine has been ascribed a regulatory effect on oligodendroglial development of second-generation antipsychotics.18 Likewise, risperidone and aripiprazole may suppress microglial activation of inflammatory substances and promote neuroprotection, 19,20 although this neuroprotective effect on white matter was not confirmed in a recent clinical trial.21
Since antipsychotics differ in receptor profiles, potential brain structural effects of individual antipsychotic compounds rather than class effects should be pursued.18,22 Although the effect of antipsychotics is tightly associated with striatal dopamine D2/3 receptor occupancy23 and dopamine regulates the neuromodulatory NMDA receptors, the effect of selective dopaminergic blockade on white matter has, to our knowledge, not previously been investigated.
By means of DTI, we investigated white matter integrity in antipsychotic-naive patients with first-episode schizophrenia and healthy controls before and after 6 weeks of antipsychotic monotherapy with the selective dopamine D2/3 receptor antagonist amisulpride. We expected subtle baseline alterations of diffusion parameters in patients compared with controls. Furthermore, we hypothesized that patients’ baseline positive symptoms would be associated with the integrity of frontal white matter fasciculi. Finally, we expected that treatment would be associated with white matter restoration.
Methods
Participants
As part of a large first-episode project, antipsychotic-naive patients with schizophrenia who were between 18 and 45 years old were recruited between December 2008 and July 2011 from psychiatric hospitals and outpatient psychiatric clinics in the capital region of Denmark. Diagnoses of schizophrenia or schizoaffective psychosis were based on ICD-10 and confirmed using the Schedule of Clinical Assessment in Neuropsychiatry (SCAN), version 2.1. Details of recruitment procedures have been reported elsewhere.10,11 We excluded patients with a current ICD-10 diagnosis of drug dependency, but previous diagnoses of drug dependency or intermittent recreational use of drugs were accepted. Current drug use was measured using a urine test (Rapid Response, Jepsen HealthCare). Lifetime methylphenidate exposure and use of antidepressants and mood stabilizers within the month preceding the study and during the treatment period were exclusion criteria. Benzodiazepines were allowed, but their use was restricted on days of examination.
We recruited healthy controls matched for age, sex and parental socioeconomic status from the community. To exclude those with former or present psychiatric illness, substance abuse or first-degree relatives with psychiatric diagnoses, all controls underwent a SCAN interview.
Upon completion of baseline examinations, all patients underwent 6 weeks of antipsychotic monotherapy with amisulpride. To avoid the need for anticholinergic medication, we meticulously adjusted individual amisulpride doses according to symptoms and adverse effects, particularly extrapyramidal symptoms and sedation.
The study was conducted in accordance with the declaration of Helsinki II and approved by the Danish National Committee on Biomedical Research Ethics (H-D-2008–088). All participants provided signed informed consent. Despite psychotic symptoms, patients were fully intellectually capable of consenting, and no coercive measures were allowed.
Clinical measures
Trained raters assessed psychopathology using the positive and negative syndrome scale (PANSS).24 Level of functioning was assessed using Global Assessment of Functioning (GAF), and depressive symptoms were assessed using the Calgary Depression Scale for Schizophrenia.25 We considered the duration of untreated illness (DUI) to be the time between a continuous invasive deterioration of functioning due to psychosis-related symptoms and the date of the baseline MRI scan.26
We assessed handedness using the Edinburgh Handedness Inventory.27
Image acquisition and processing
We obtained MRI scans using a Philips Achieva 3.0 T whole body MRI scanner (Philips Healthcare) with an 8-channel SENSE head coil (Invivo). Whole brain DTI images were acquired using single shot spin-echo echo-planar imaging (EPI) and a total of 31 different diffusion encodings (5 diffusion-unweighted [b = 0 s/mm2] and 30 diffusion-weighted [b = 1000 s/mm2] noncollinear directions). To enable correction for susceptibility distortions, acquisition of each volume was repeated with the opposite phase encoding direction under the following parameters: acquired matrix size 128 × 128 × 75, voxel dimensions 1.88 × 1.88 mm × 2 (no slice gap), repetition time (TR) 7035 ms, echo time (TE) 68 ms, flip angle 90°.
Image processing was based on the FSL library of tools (http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/fsl/list.html). The EPI sequences underwent eddy current correction.28 Using affine registration, head motion was corrected to a reference volume followed by correction of susceptibility distortions.29 Next, data were interpolated to 1.33 × 1.33 × 2 voxel dimensions using sinc interpolation. We removed nonbrain tissue using a brain extraction tool (BET).30 Diffusion parameter maps of the primary measure, fractional anisotropy (FA), and the secondary measures, mean diffusivity (MD), parallel diffusivity (λ1) and radial diffusivity (λ23), were derived using DTIFIT.
Whole brain
In the primary analyses, we assessed voxelwise group differences using tract-based spatial statistics (TBSS),31 which estimates the central integrity of the main fibre tracts and is therefore supreme for nonbiased whole brain analyses. First, FA data were nonlinearly registered using FNIRT onto the standard FMRIB58 FA template.32 Next, the nearest maximum FA values of each registered FA image were projected onto a white matter skeleton derived from the FMRIB58 template and thresholded at FA = 0.2.
Frontal fasciculi
In the secondary analyses, we addressed the hypotheses of delayed corollary discharges by deriving anatomic regions of interest (ROIs) of 8 mutually exclusive frontal fasciculi: the left and right uncinate fasciculus (UF), left and right superior longitudinal fasciculus (SLF), left and right cingulum (CG) and left and right arcuate fasciculus (AF).9
We chose the ROI approach to take advantage of its higher regional sensitivity as compared with a whole brain voxel-based method. As opposed to TBSS, the ROI method estimates the integrity of entire ROIs, rather than the central integrity of the tract only. The anatomic ROIs of the UF, SLF and CG were obtained from JHU ICBM-DTI-81 atlas white matter labels. The ROI of the AF was obtained from www.natbrainlab.com. We calculated the probability-weighted mean FA of the ROIs by multiplying the probability-weighted ROIs with FA maps and then extracting the mean FA within each of the anatomic ROIs.
Statistical analysis
Demographic and clinical data
We used Statistical Package for the Social Sciences software version 20 (SPSS Inc.) to analyze demographic and clinical data. The distribution of continuous data was tested for normality using the Shapiro–Wilk test. Because data on age and weight were not normally distributed, group comparisons were performed nonparametrically using the Mann–Whitney U test. We assessed handedness and sex differences using the Fisher exact test and socioeconomic status using the Pearson χ2 test. Within patients, changes in PANSS scores were tested using a paired-samples t test.
Voxelwise statistical analyses
The exploratory voxelwise group comparisons, time effects and interactions on the skeletonized baseline images were estimated nonparametrically with general linear models using Randomize version 2.1, which is part of the FSL library of tools (http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/randomise/index.html), with 5000 permutations using age and sex as covariates. Family-wise error (FWE) correction with a threshold of p < 0.05 using threshold-free cluster enhancement33 was applied to correct for multiple comparisons.34 Anatomic locations of significant ROIs (“cluster ROIs”) were identified in Montreal Neurological Institute (MNI) space using the Johns Hopkins University (JHU) white matter tractography atlas in FSL.35,36
Next, we averaged diffusion parameters across each cluster ROI. We used STATA software version 13.1 (StataCorp) for regression analyses comprising the ordered probit model37 and repeated-measures analysis of variance (ANOVA). Progressive changes in cluster ROIs were analyzed using repeated-measures ANOVA. The FA values at baseline and follow-up were analyzed separately within the patient group using the ordered probit model with PANSS scores (positive, negative, general) as a dependent variable and all cluster ROIs as predictor variables. We used ordinal regression analyses because our hypothesis specifically implied associations with the PANSS positive subscore, consisting of 7 ordinal variables.37
Additional DTI parameters, MD, λ1, and λ23, were extracted from identified cluster ROIs and used only in secondary analyses to explore which parameters were driving any significant FA changes.
Anatomic ROI-based statistical analyses
Probability-weighted mean FA within the 8 anatomic ROIs defined a priori was used for linear, ordinal regression analyses and repeated-measures ANOVA using STATA. We used a linear regression model to test for baseline group differences in FA. To explore progressive FA changes in the anatomic ROIs over 6 weeks, we used repeated-measures ANOVA (group × time).
Further, we tested the hypothesized associations between clinical variables and FA changes in the patient group. The probability-weighted mean FA of the anatomic ROIs at baseline and follow-up were entered into ordinal regression analyses using ordinal PANSS (positive, negative, general) scores as the dependent variable and continuous FA values of all 8 anatomic ROIs as predictor variables. For visualization, the contribution from individual predictor variables was estimated using multiple linear regression in STATA.
All tests were 2-tailed, and the significance level was set to p < 0.05. Because our primary hypothesis was to test FA correlations with psychopathology, p values were corrected for the number of PANSS subscores (positive, negative, general), resulting in a Bonferroni-corrected p value of 0.017. Only findings that survived correction for multiple comparisons are reported. All group comparisons were corrected for age and sex. Post hoc we performed analyses correcting for years of schooling, smoking and weight change.21 Because metabolic changes have recently been suggested to influence FA,21 we performed post hoc analyses corrected for weight changes in the patient group.
Results
Participants
We included 38 patients and 38 controls in our study, and 28 participants in each group completed the study (the reasons for attrition are described later in the Results section). No participants had any history of major head injury, and physical and neurologic examination findings were normal. The MRI scans were without overt pathology, as evaluated by a trained neuroradiologist. The baseline demographic and clinical characteristics of study participants are provided in Table 1 and Table 2. Patients and controls were well matched on age, sex and parental socioeconomic status. There were no group differences in height, weight or handedness. Patients had significantly fewer years of education than controls, and more patients than controls were smokers (Table 1).
Baseline demographic characteristics of study participants
Clinical characteristics of patients at baseline and at 6-wk follow-up
Baseline
Voxelwise whole brain analyses
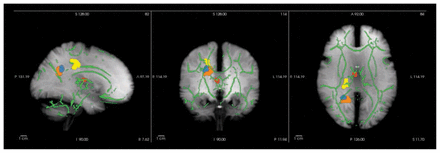
Baseline voxelwise comparisons of FA maps revealed 4 significant cluster ROIs in which patients had lower FA than controls: the right anterior thalamic radiation (ATR), right cingulum (CG), right inferior longitudinal fasciculus (ILF) and right corticospinal tract (CT; Fig. 1).
Whole brain voxelwise group differences visualized using FreeSurfer (https://surfer.nmr.mgh.harvard.edu/). Clusters are enhanced using tbss_fill (http://fsl.fmrib.ox.ac.uk/fsl/fsl4.0/tbss/index) and projected on the tract-based spatial statistics skeleton (green) and a maximum intensity projection of a T1-weighted image (grey). Patients display fractional anisotropy reductions in the right anterior thalamic radiation (red), right cingulum (yellow), right inferior longitudinal fasciculus (blue) and right corticospinal tract (orange). See Table 3 for specifications.
Ordinal regression analyses of cluster ROIs (pseudo-R237 = 0.089, p = 0.012) showed that FA in the right ATR correlated positively with positive symptoms (pseudo-R236 = 0.067, z = 2.64, p = 0.008; Table 3).
Specifications of whole brain voxelwise group differences localized by FSL
Two-sample t tests of secondary DTI parameters in these cluster ROIs revealed that lower FA values corresponded to lower λ1 values in the CG (t74 = 3.62, p < 0.001) and ILF (t74 = 3.33, p = 0.001). Moreover, λ23 values were higher in the ATR (t74 = −2.6, p = 0.009), CG (t74 = −2.4, p = 0.018) and CT (t74 = −2.2, p = 0.026).
Post hoc analyses restricted to the 28 patients and 28 controls who completed the study did not reveal significant group differences (all p > 0.18). The loss of significance appeared to be explained by loss of power, as determined by visual inspection of plots (data not shown). The correlation between the right ATR and positive symptoms remained significant.
Anatomical ROI-based analyses
The repeated-measures general linear model (with 2 hemisphere levels and 4 frontal fasciculi ROI levels), did not reveal differences between patients and controls. However, we found a significant main effect of age (z = −2.33, p = 0.020).
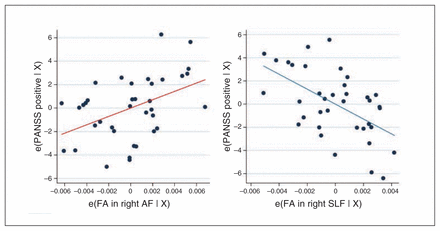
In the planned ordinal regression analyses of the frontal fasciculi in patients, we, as predicted, found a significant association between PANSS positive symptoms and FA of the anatomic ROIs (pseudo-R237 = 0.15, p = 0.002). This association was driven by positive correlation with FA in the right AF (z = 2.83, p = 0.005) and by a negative correlation with FA in the right SLF (z = −3.31, p = 0.001; Fig. 2).
Added-variable plots between residuals of baseline Positive and Negative Syndrome Scale (PANSS) positive scores and fractional anisotropy (FA) within the right arcuate fasciculus (AF) and right superior longitudinal fasciculus (SLF) after adjusting for all other predictors. (A) The scatter plot of the residuals shows positive correlation between the right AF and PANSS positive scores (red regression line). (B) The scatter plot of the residuals shows negative correlation between the right SLF and PANSS positive scores (blue regression line). e(FA in right AF | X) = residuals after fitting the reduced model (model without FA in the AF) to FA in the AF; e(FA in right SLF | X) = residuals after fitting the reduced model (model without FA in the SLF) to FA in the SLF; e(PANSS positive | X) = residuals after fitting the reduced model (model without FA in the AF/SLF) to PANSS positive scores.
We found no associations between PANSS negative, PANSS general, PANSS total, GAF, Calgary Depression Scale or DUI scores. No associations between baseline FA and changes in clinical measures emerged. Likewise, no associations between baseline FA and clinical measures at follow-up were found.
Post hoc analyses restricted to participants who completed the study did not alter our findings.
Follow-up
Demographics and attrition
During the 6-week treatment period 10 of the 38 patients dropped out, corresponding to an attrition rate of 26%. The reasons for attrition were refusal to take medication or lack of compliance (n = 6), panic attack at second MRI session (n = 1), intolerable side-effect (galactorrhea; n = 1) and change to another antipsychotic compound (n = 2). Thus, 28 patients and 28 healthy controls were included in the follow-up analyses (Table 2). The mean dose of amisulpride (262 ± 177 [range 100–800] mg/d) did not correlate with baseline or follow-up PANSS scores or other clinical variables.
Participants who dropped out had significantly more years of education than those who completed the study (13.5 ± 2.4 v. 11.6 ± 2.4, t = −2.08, p = 0.044). Baseline characteristics of participants who dropped out and those who completed the study did not differ with regard to age; handedness; parental socioeconomic status; smoking habits; weight; DUI, GAF, and Calgary Depression Scale for Schizophrenia scores; or PANSS positive, negative, general or total scores.
Voxelwise whole brain analyses of progressive changes
The repeated-measures ANOVA within the 4 cluster ROIs, revealed a significant interaction: FA in the right ATR increased more in patients than in controls during the 6-week treatment phase (z = −4.92, p < 0.001). This effect was mainly driven by decreases in λ23 (z = 3.12, p = 0.002) in patients. In addition, linear regression analyses revealed a positive correlation between amisulpride dose and increase in FA in the right CT (t = 2.52, p = 0.019).
No significant correlations between changes in FA in the cluster ROIs and changes in clinical variables or in follow-up clinical variables were observed (Table 3). Voxelwise analyses of the whole brain at follow-up did not reveal significant clusters indicative of interactions or time effects.
Correction for years of education, smoking and weight change within patients did not significantly alter the correlations between FA and psychopathology. A linear regression analysis revealed significant effects of weight change on FA changes in the CT (t = −2.96, p = 0.007).
Anatomical ROI-based analyses of progressive changes
No longitudinal or follow-up group differences in any of the anatomic ROIs of the frontal fasciculi were found. Likewise, we found no longitudinal or follow-up correlations between clinical measures and FA changes within the anatomic ROIs.
Correcting for years of education, smoking and weight change and did not significantly change the results of group comparisons. However, we found significant effects of weight change on progressive FA changes in the anatomic ROIs of the left AF (t = −3.03, p = 0.006) and left SLF (t = −2.85, p = 0.009).
Discussion
In the present study, we investigated the integrity of white matter in antipsychotic-naive patients with schizophrenia before and after 6 weeks of antipsychotic monotherapy with amisulpride.
Whole brain voxelwise analyses revealed reduced FA values in the right ATR and other posteromedial brain regions in patients compared with controls. The FA deficits in the right ATR positively correlated with positive symptoms ( Table 3). Because the ATR contains fibre pathways that connect the anterior and midline nuclear groups of the thalamus with the frontal lobe,38 the ATR in the current context can be considered a frontal fasciculus.
The absence of group differences in FA of the frontal fasciculi ROIs underlines that the white matter alterations in the early stage of schizophrenia are subtle and stresses the argument for supplementing with ROI analyses for increased regional sensitivity. As such, interpretations of ROI results may be strengthened by taking into account the presence of whole brain group differences.
As predicted, we found a significant association between severity of positive symptoms and FA in the anatomic ROIs of the frontal fasciculi. As we found no other baseline associations between FA in the frontal fasciculi and clinical variables (PANSS negative, general and total scores; GAF, Calgary Depression Scale and DUI scores), these findings appear specific to positive symptoms.
Because the white matter aberrations were observed in widespread regions of the brain (Fig. 1), this could indicate an unspecific disease effect along long-range white matter tracts. However, all associations between positive symptoms and FA appeared in long-range association tracts projecting into the frontal lobe. Thus our findings lend support to the hypothesis of delayed corollary discharges underlying psychosis.8,9
We found a positive correlation between positive symptoms and FA in the right AF and a negative correlation between positive symptoms and FA in the right SLF (Fig. 2). Curiously, a recent meta-analysis found that FA in left AF was negatively correlated with auditory verbal hallucinations.39 These discrepancies in findings may be explained by the fact that our patients were antipsychotic-naive and that PANSS positive symptoms cover several items other than auditory verbal hallucinations.
Intuitively, symptom severity would be negatively correlated with FA; however, other DTI studies have reported positive correlations between positive symptoms and FA.40–42 Moreover, recent functional MRI studies have indicated that functional connectivity in the prefrontal cortex increases with the severity of psychotic symptoms, especially in the early stage of schizophrenia.43,44 As functional hyperconnectivity may be reflected in regional FA increases,40–42 our findings of both positive and negative associations with positive symptoms in 2 closely linked anatomical structures (AF and SLF) support the idea that psychotic symptoms arise from regional irregularities in white matter rather than deficits per se.
Clinically, the notion of white matter irregularities, which was incorporated in the original hypothesis on dysconnection in schizophrenia,8 implies a biological complexity behind symptoms that may compromise potential effects of remyelinating medication as a new treatment strategy for psychosis.9
In accordance with a previous study involving voxelwise analyses in a comparable sample, all significant FA reductions in our patients were located in the right hemisphere.45 Exploratory application of a discretely more liberal threshold (e.g., pFWE < 0.06) also revealed left-sided group differences, suggesting that disease-related changes in white matter may be more prominent in but are not restricted to the right side.
Three of our 4 identified cluster ROIs (ATR, CG and ILF) have been associated with schizophrenia on a meta-analytical level.46 The aforementioned meta-analysis found that chronicity and negative symptoms were associated with severity of white matter deficits.46 As we did not observe associations between DUI, negative symptoms and FA reductions ( Table 3), our findings support the idea that only subtle white matter deficits in these regions are present at illness onset, but that these deficits disseminate and progress over time.47 Our observation of FA reductions in the right CT is in line with findings in patients with early-onset schizophrenia and in individuals at high risk for psychosis.48 However, deficits in this region are less frequently observed in later stages of illness, suggesting that FA changes in the CT may be more prominent at the time of illness onset.
Six weeks of amisulpride treatment significantly reduced positive, general and total PANSS scores (Table 2), and at follow-up all correlations between positive symptoms and frontal fasciculi (including the ATR) had resolved. The reduced variations in positive symptom scores partly explain this finding. However, independent of symptoms scores, we observed a positive correlation between amisulpride dose and increase in FA in the right CT. Moreover, the improvement in the right ATR appeared driven by a decrease in radial diffusivity, λ23. As radial diffusivity is considered to be particularly sensitive to myelination,49,50 our observation could suggest a subtle protective effect of D2/3 blockade on white matter. Changes in mean diffusivity and parallel diffusivity did not survive correction for multiple comparison (data not shown). We observed no indications of a negative impact of amisulpride treatment on white matter deficits. On the contrary, at follow-up FA values were generally numerically higher. In line with 2 recent longitudinal studies over 12 weeks that indicated clozapine treatment51 and mixed antipsychotic treatment52 improved white matter integrity, our findings support that second-generation antipsychotics may protect against myelin degradation19,20 or even improve myelination.53–55 Specifically, and consistent with previous observations of functional hyperfrontality in patients with first-episode schizophrenia, our findings could indicate that selective dopamine D2/3 blockade regulate the NMDA receptors, which in turn reduce the putatively elevated and neurotoxic levels of glutamate associated with psychosis.56
A strength of the present study is the longitudinal design of initially antipsychotic-naive patients exposed to antipsychotic monotherapy with a selective D2/3 receptor profile. The anatomic ROIs of the frontal fasciculi were selected a priori and based on a hypothesis proposed by an independent research group.9
Methodologically, we analyzed the diffusion data using a well-validated approach (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS). To minimize the risk for type I errors, we focused analyses on FA, which is considered a conservative and general measure of white matter integrity. Moreover, we have exclusively reported results that survived correction for multiple comparisons.
Limitations
To enhance the external validity and increase the inclusion rate, a previous diagnosis of substance abuse was allowed in patients but not in controls. Also, more patients than controls were smokers (Table 1). Although smoking (number of cigarettes/d) did not significantly correlate with FA in patients or controls, these factors may still have influenced our findings.57,58
Twenty-six per cent of the patients dropped out during follow-up, and those who dropped out had more years of education than those who did not drop out. As correction for education did not alter our results, we consider it unlikely that baseline differences substantially influenced our results.
Because we did not include a study arm of patients treated with an antipsychotic compound with less pronounced D2/3 receptor blockade (e.g., quetiapine), our observations of potential remyelinating effects of amisulpride could represent unspecific treatment effects rather than D2/3 receptor blockade per se. Moreover, the mean dose of amisulpride in the present study (272 mg/d) was lower than in other first-episode studies (e.g., the EUFEST study, which used a dose of 451 mg/d over 12 mo).59 In both studies, the clinical symptoms improved, but in contrast to the EUFEST study, we avoided prescriptions for anticholinergic medication. Finally, our follow-up period of 6 weeks limits interpretations of potential long-term effects of antipsychotic treatment on white matter.
As an expected side-effect, patients experienced susbtantial weight gain during the study period (Table 2). Consistent with a previous study,21 we found an effect of weight change and FA changes in several ROIs; however, correlations with psychopathology were unaffected. As obesity has been associated with compromised white matter integrity in otherwise healthy individuals,60 our findings encourage further exploration of associations between diffusion parameters and metabolic side effects of antipsychotics.
Conclusion
Antipsychotic-naive patients with schizophrenia display subtle deficits in white matter, and psychotic symptoms appear to be associated specifically with frontal fasciculi integrity. Six weeks of amisulpride treatment may normalize white matter integrity. Potential remyelinating effects of dopamine D2/3 receptor antagonism warrant further clarification.
Footnotes
↵* These authors contributed equally to this work.
Funding: This work was supported by a grant from the University of Copenhagen/Mental Health Services, Capital Region of Denmark awarded to B. Ebdrup. The Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research, CINS is funded by Lundbeck Foundation grant number R25-A2701.
Competing interests: B. Ebdrup declares lecture fees from Bristol-Myers Squibb, Otsuka Pharma Scandinavia AB and Eli Lilly, and he is on the advisory boards of Eli Lilly Danmark A/S and Takeda Pharmaceutical Company Ltd. No other competing interests declared.
Contributors: B. Ebdrup, J. Raghava, E. Rostrup and B. Glenthoj designed the study. M. Nielsen acquired the data, which B. Ebdrup, J. Raghava, E. Rostrup and B. Glenthoj analyzed. B. Ebdrup and J. Raghava wrote the article, which all authors reviewed and approved for publication.
- Received January 30, 2015.
- Revision received May 27, 2015.
- Accepted July 14, 2015.