Abstract
Background: Prader Willi syndrome is a genetic disorder with a behavioural expression characterized by the presence of obsessive–compulsive phenomena ranging from elaborate obsessive eating behaviour to repetitive skin picking. Obsessive–compulsive disorder (OCD) has been recently associated with abnormal functional coupling between the frontal cortex and basal ganglia. We have tested the potential association of functional connectivity anomalies in basal ganglia circuits with obsessive–compulsive behaviour in patients with Prader Willi syndrome.
Methods: We analyzed resting-state functional MRI in adult patients and healthy controls. Whole-brain functional connectivity maps were generated for the dorsal and ventral aspects of the caudate nucleus and putamen. A selected obsessive–compulsive behaviour assessment included typical OCD compulsions, self picking and obsessive eating behaviour.
Results: We included 24 adults with Prader Willi syndrome and 29 controls in our study. Patients with Prader Willi syndrome showed abnormal functional connectivity between the prefrontal cortex and basal ganglia and within subcortical structures that correlated with the presence and severity of obsessive–compulsive behaviours. In addition, abnormally heightened functional connectivity was identified in the primary sensorimotor cortex–putamen loop, which was strongly associated with self picking. Finally, obsessive eating behaviour correlated with abnormal functional connectivity both within the basal ganglia loops and between the striatum and the hypothalamus and the amygdala.
Limitations: Limitations of the study include the difficulty in evaluating the nature of content of obsessions in patients with Prader Willi Syndrome and the risk of excessive head motion artifact on brain imaging.
Conclusion: Patients with Prader Willi syndrome showed broad functional connectivity anomalies combining prefrontal loop alterations characteristic of OCD with 1) enhanced coupling in the primary sensorimotor loop that correlated with the most impulsive aspects of the behaviour and 2) reduced coupling of the ventral striatum with limbic structures for basic internal homeostasis that correlated with the obsession to eat.
Introduction
Prader Willi syndrome is a genetic disorder with an estimated prevalence of 1 in 10 000–30 000 caused by chromosome 15 long arm anomalies. The syndrome combines intellectual disability with characteristic physical, endocrine and behavioural traits.1 The behavioural expression of the syndrome is broad and marked by obsessive–compulsive phenomena, including food-related obsessions and compulsive eating, compulsive ordering, hoarding and repetitive skin picking.2–7 Phenomenologically, however, it is not obvious the extent to which the obsessive–compulsive features of Prader Willi syndrome overlap with the characteristic symptoms of obsessive–compulsive disorder (OCD). Obsessional thoughts per se seem less apparent than compulsions in patients with Prader Willi syndrome, and some typical OCD compulsions, such as ritualized hand washing and checking, are not common.2,4 Other behavioural features of Prader Willi syndrome include high levels of impulsivity, response perseveration, motor stereotypies, repetitive speech and temper tantrums.2,3,8,9
Imaging studies of typical patients with OCD have demonstrated a dysfunctional coupling in frontal–basal ganglia circuits. The core alteration involves enhancement of functional connectivity between the prefrontal cortex and the ventral striatum that co-occurs with an opposite pattern of reduced connectivity in surrounding cortical areas.10,11 The morphology of the frontobasal systems is also subtly altered in patients with OCD.12–14 In patients with Prader Willi syndrome, measurements of brain regional tissue volume,15,16 perfusion,17,18 glucose metabolism,19 anatomic connectivity,20 resting activity21 and brain response to food stimuli22–25 have all shown some degree of anomaly in the frontobasal brain. The orbitofrontal cortex was found to be altered in most studies, but the data indicate that the changes may implicate each frontal–basal ganglia circuit and may even extend to anterior temporal lobe structures. However, it is relevant that the potential association of altered basal ganglia circuit function with obsessive–compulsive behaviour has not been examined in patients with Prader Willi syndrome.
The present study was developed to assess functional connectivity in basal ganglia circuits in patients with Prader Willi syndrome using an equivalent approach to that previously adopted to study patients with OCD.10 We tested both the functional status of the circuits by comparing patients with Prader Willi syndrome and healthy controls and by studying the potential association of functional connectivity alterations with the presence and severity of selected obsessive–compulsive behaviour (i.e., typical compulsions, self picking and food-related obsessive–compulsive behaviour). Our hypothesis was that Prader Willi syndrome would be associated with enhanced cortico-subcortical functional connectivity, as seen in patients with OCD, but we predicted broader basal ganglia circuit disturbances in keeping with its characteristically diverse behaviour-control deficiencies.
Methods
Participants
We recruited adults with Prader Willi syndrome from the Genetic, Pediatrics and Endocrinology Departments of Corporació Sanitària Parc Taulí of Sabadell, Barcelona, Spain (the clinical referral centre for Prader Willi syndrome) and the Specialized Mental Health and Intellectual Disability Service, Parc Hospitalari Martí i Julià of Salt, Girona, Spain. The Catalan Association of Prader Willi Syndrome of Barcelona and the Prader Willi Syndrome Foundation of Madrid also assisted with recruitment. Patients younger than 18 years, those who had nonstable medical conditions and those considered unable to follow MRI instructions were not eligible to participate in the study.
Genetic testing to confirm the chromosome 15 anomaly was repeated in all patients at the time of inclusion in the study. We collected blood samples for genomic DNA extraction and for karyotype. In each case, Prader Willi syndrome was confirmed by methylation-specific polymerase chain reaction (MS-PCR) with an absence of paternal allele at 15q11-q13, and the deletion status was established by fluorescence in situ hybridization (FISH). For patients with the deletion, we identified the subtype designation (class 1 or class 2) using methylation-sensitive multiplex ligation dependent probe amplification (MS-MLPA; MRC-Holland). In addition, microdeletion of the imprinting centre (IC) could be identified. In the absence of a deletion, analysis of multiple microsatellite markers distributed inside the 15q11-q13 region and along chromosome 15 in the proband and their parents were performed to distinguish maternal uniparental disomy (UPD). When a biparental inheritance was identified the UPD was excluded and the diagnosis was a sporadic epigenetics imprinting defect.
We also recruited a group of healthy controls matched by age and sex to the initial patient sample. Control participants underwent a complete medical and psychiatric assessment, including a comprehensive interview with a senior psychiatrist and the obsessive–compulsive testing administered to patients. Individuals with relevant medical or neurologic disorders, substance abuse or psychiatric disease and those undergoing medical treatment were not considered eligible for inclusion in the study.
We conducted this study according to the principles expressed in the Declaration of Helsinki. The protocol was approved by the Clinical Research Ethical Committee of the Corporació Sanitària Parc Taulí of Sabadell, Barcelona. We obtained written informed consent from the parents of patients and from control participants. Verbal or written consent was also obtained from the patients with Prader Willi syndrome.
Obsessive–compulsive behaviour assessment
To identify the presence and severity of characteristic symptoms related to obsessive–compulsive behaviour in patients with Prader Willi syndrome, their caregivers completed assessments within the 2 weeks before the fMRI scan (mean 7 d). The Compulsive Behaviour Checklist26 was used to confirm the presence of various compulsive symptoms grouped in several categories: ordering, completeness/incompleteness, cleaning/tidiness, checking/touching and deviant grooming. The deviant grooming category is a self picking equivalent27 that assesses body-focused repetitive behaviour: skin picking, hair pulling/cutting and obsessive checking of body parts.26 A previous factor analysis clearly differentiated deviant grooming/self picking from the other compulsions of the Compulsive Behaviour Checklist.27 The Yale–Brown Obsessive Compulsive Scale (Y-BOCS)28 was used to provide a complementary assessment of the severity of target compulsions identified using the Compulsive Behaviour Checklist.
The Hyperphagia Questionnaire29 is a 13-item instrument specifically designed to measure food-related preoccupations and problems in patients with Prader Willi syndrome as well as the severity of these concerns. The items measure hyperphagic symptoms reported by relatives and are rated on a 5-point scale (1 = not a problem; 5 = severe and/or frequent problem). It gives a total score as well as 3 factorial analytic subscale scores across the dimensions of behaviour, drive and severity.
MRI acquisition
We used a 1.5 T Signa Excite system (General Electric) equipped with an 8-channel phased-array head coil and single-shot echoplanar imaging (EPI) software to obtain MRI scans. The functional sequence consisted of gradient recalled acquisition in the steady state emphasizing blood oxygen level–dependent (BOLD) contrast (repetition time 2000 ms, echo time 50 ms, pulse angle 90º, field of view 24 cm, 64 × 64-pixel matrix, slice thickness 4 mm, interslice gap 1.5 mm). Twenty-two interleaved slices were prescribed parallel to the anterior–posterior commissure line covering the whole brain. We acquired a 6-min continuous resting-state scan for each participant in the morning before breakfast. This scan generated 180 whole-brain EPI volumes. The first 4 (additional) images in each run were discarded to allow magnetization to reach equilibrium. Participants were instructed to relax, stay awake and lie still without moving while keeping their eyes closed throughout the scan. Participants were systematically questioned about their arousal level during the resting-state assessment immediately after the acquisition. We obtained self-report confirmation of wakefulness from each participant.
Image preprocessing
Imaging data were processed using MATLAB version 2011b (the MathWorks Inc.) and Statistical Parametric Mapping software (SPM8; The Wellcome Department of Imaging Neuroscience). Preprocessing involved motion correction, spatial normalization and smoothing using an 8 mm full-width at half-maximum (FWHM) Gaussian filter. Data were normalized to the standard SPM-EPI template and resliced to 2 mm isotropic resolution in Montreal Neurological Institute (MNI) space. All image sequences were inspected for potential acquisition and normalization artifacts.
Control of potential head motion effects
To control for the effects of head motion, we adopted the following procedures. Participants with large head motion (boxplot-defined outliers)30 were excluded. Time series were aligned to the first image volume in each participant using a least squares minimization and a 6-parameter (rigid body) spatial transformation. We included 12 motion-related regressors and estimates of global brain signal fluctuations as confounding variables in our first-level (single-subject) analyses. Within-subject, censoring-based MRI signal artifact removal31 (scrubbing) was used to discard motion-affected volumes. For each participant, interframe motion measurements30 served as an index of data quality to flag volumes of suspect quality across the run. At points with interframe motion > 0.2 mm, we discarded that corresponding volume, the immediately preceding and the succeeding 2 volumes. Using this procedure, a mean of 8.6 (5%) volumes (range 0–75) from the total 180 volumes that are included in the fMRI sequence were removed in patients and a mean of 1.3 (1%) volumes (range, 0–17) were removed in controls. Finally, potential motion effects were removed using a summary measurement for each participant (mean interframe motion across the fMRI run) as a regressor in the second-level (group) analyses in SPM.30
Functional connectivity analysis
We generated functional connectivity maps, adapting the procedures detailed by Harrison and colleagues10 and Di Martino and colleagues,32 and focused on the segregation of functional connectivity maps between the dorsal and ventral striatum. In this approach, dorsal and ventral striatal subregions were distinguished using z < 7 mm as a marker for the ventral caudate/nucleus accumbens, z > 7 mm as a marker for dorsal caudate and z = 2 as the boundary between the dorsal and ventral putamen. We obtained a total of 8 maps by locating the seed regions at the dorsal and ventral aspects of the caudate nucleus and putamen in both brain hemispheres (dorsal caudate nucleus: x(±), y, z = 13, 15, 9; dorsal putamen: x(±), y, z = 28, 1, 3; ventral caudate nucleus, corresponding approximately to the nucleus accumbens: x(±), y, z = 9, 9, −8; ventral putamen x(±), y, z = 20, 12, −3). Appendix 1, Figure S1, available at jpn .ca, illustrates the anatomic location of the seeds. We did not use the third intermediate seed defined by Di Martino and colleagues32 to further subdivide the striatum in order to focus more simply on striatal dorsal/ventral divisions, showing good face validity in human functional connectivity mapping studies, and to be consistent with previous work on OCD.10,11
For each of the striatal locations, the seed region was defined as a 3.5 mm radial sphere (sampling ~25 voxels in 2 mm isotropic space) with a minimum Euclidean distance of 8 mm between any 2 regions. This was performed using the MarsBaR region of interest (ROI) toolbox in MNI stereotaxic space.33 Signals of interest were then extracted for each seed region respectively by calculating the mean ROI value at each time point across the time series.
To generate the seed maps, we used the signal time course of a selected seed region as a regressor to be correlated with the signal time course of every voxel in the brain in order to generate individual voxel-wise statistical parametric maps of functional connectivity. The maps were estimated for each seed separately. A high-pass filter set at 128 s was used to remove low-frequency drifts below approximately 0.008 Hz. In addition, we derived estimates of white matter, cerebrospinal fluid and global brain signal fluctuations to include in the regression analyses as nuisance variables. The first level of our statistical analysis, therefore, involved the generation of single-subject brain maps of functional connectivity expressed as β regression estimates.
Single-subject voxel-wise functional connectivity maps were then included in second-level (group) random-effects analyses to test for group effects (1-sample t tests) and to compare the groups (2-sample t tests). The 1-sample t tests served to map brain regions showing significant functional connectivity within a given group (connectivity > 0). The 2-sample t tests served to map significant functional connectivity differences for the patients > controls contrast and the patients < controls contrast. Each hypothesis was addressed with 1-sided tests. Voxelwise analyses in SPM were also performed to map the correlation between resting-state functional connectivity and selected (the most prevalent in this sample) obsessive–compulsive symptoms (i.e., number of “ordering” compulsions, self picking, compulsion severity according to Y-BOCS, and Hyperphagia Questionnaire total score and “severity” subscore).
Thresholding criteria
Spatial extent thresholds were determined based on 2000 Monte Carlo simulations using AlphaSim, as implemented in the SPM REST toolbox.34 Input parameters to AlphaSim included an individual voxel threshold probability of 0.005, cluster connection radius of 5 mm, 8 mm FWHM smoothness, incorporating a grey matter mask volume of 167 265 (2 × 2 × 2 mm) voxels. Results were considered significant with clusters of 1.032 mL (129 voxels) at a height threshold of p < 0.005, which satisfied the family-wise error (FWE) rate correction of pFWE < 0.05. All maps in figures are displayed at t > 2.4.
Results
Participants
We recruited 30 adults with Prader Willi syndrome and 30 controls for participation in the study. Six patients and 1 control were excluded on the basis of excessive head motion during fMRI. These exclusions resulted in a final sample of 24 patients (12 women, 12 men, mean age 26.3 ± 6.9 [range 18–39] yr) with genotype-confirmed anomaly of chromosome 15 and 29 controls (14 women, 15 men, mean age 28.2 ± 7.7 [range 19–45] yr). The excluded individuals did not differ from the rest of the sample in age, sex distribution or any behavioural measurement. The demographic and clinical characteristics of the sample are reported in Table 1. The Prader Willi group included 3 patients with type 2 diabetes mellitus (2 receiving oral agents and 1 insulin) and 6 with dyslipidemia. A total of 13 patients were taking psychiatric medication (fluoxetine, topiramate, or both, occasionally combined with antipsychotics [n = 4]). Treated patients were on a stable medication regime for at least 3 months prior to imaging assessment.
Demographic and clinical characteristics of study participants
Functional connectivity maps
In both groups, basal ganglia functional connectivity maps corresponded to well-defined networks with a cortical component involving the frontal and parietal cortex and a subcortical component involving the bilateral striatum, globus pallidus, diencephalon (thalamus, hypothalamus and subthalamus), upper mesencephalon and the amygdala (Appendix 1, Tables S1–S4 and Figs. S2–S5). At the cortical level, the caudate nucleus was more densely connected with relatively large portions of the medial, lateral and orbital prefrontal cortex and the rostral aspect of anterior cingulate cortex (ACC), whereas the putamen was more functionally connected to the sensorimotor cortex around the central sulcus and supplementary motor area (SMA).
Between-group differences
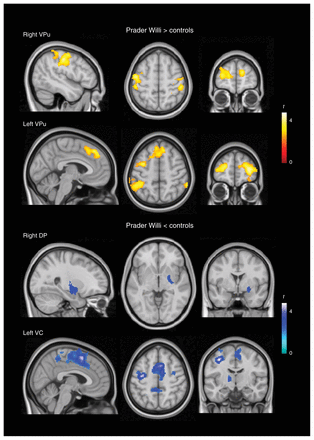
Compared with controls, patients with Prader Willi syndrome showed significantly stronger functional connectivity between the putamen and the postcentral gyrus, caudal (primary motor cortex) and rostral (premotor cortex) precentral gyrus, dorsal prefrontal cortex and anterior prefrontal cortex (frontal pole). By contrast, patients showed significantly weaker functional connectivity between the caudate nucleus and superior parietal cortex, the postcentral gyrus (somatosensory cortex), caudal and rostral precentral gyrus, SMA and orbitofrontal cortex. Within the basal ganglia, patients showed weaker functional connectivity between the (right dorsal) putamen and the globus pallidus, and between the (left ventral) caudate nucleus and the thalamus/globus pallidus (Fig. 1 and Appendix 1, Tables S1–S4). We conducted an additional analysis using body mass index (BMI) as a potential confounding variable. The results indicated that the between-group differences in functional connectivity were mostly unrelated to differences in BMI (Appendix 1, Figs. S6 and S7). Similarly, patients taking psychiatric medication (n = 13) and those not taking medication (n = 11) showed no significant functional connectivity differences in any basal ganglia circuit.
Between-group differences in basal ganglia functional connectivity. The right hemisphere corresponds to the right side of axial and coronal views. DP = dorsal putamen; VC = ventral caudate nucleus; VPu = ventral putamen.
Correlation maps in patients
Patients with Prader Willi syndrome were characterized by relevant and varied obsessive–compulsive behaviour ( Table 1). It is relevant to note the measurements showed a wide range of severity, indicating a different expression of the obsessive–compulsive behaviour across patients, which is generally consistent with the data reported in the literature (for reviews, see the studies by Dykens and Shah,3 Ho and-Dimitropoulos,4 and Stein and colleagues8). Appendix 1, Figure S8 shows the frequency distribution for the variables used in the correlation analysis.
Ordering compulsions
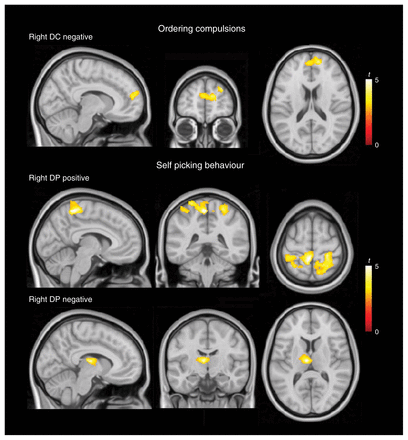
Ordering compulsions were negatively correlated with functional connectivity between the (right dorsal) caudate nucleus and the medial prefrontal cortex in its more anterior aspect (Fig. 2 and Appendix 1, Table S5).
Correlation analysis results for ordering compulsions and self picking behaviour. Top: negative correlation of ordering compulsions with functional connectivity between the dorsal caudate nucleus (DC) and the medial prefrontal cortex. Middle: positive correlation of self picking behaviour with connectivity between the dorsal putamen (DP) and somatosensory cortices. Bottom: negative correlation of self picking behaviour with connectivity between the dorsal putamen (DP) and the anterior thalamus/globus pallidus. The right hemisphere corresponds to the right side of axial and coronal views.
Self picking
Self picking behaviours were positively correlated with connectivity between the putamen and the somatosensory cortex bilaterally at the cortical representation of the trunk, inferior limb and perineal body region in the postcentral gyrus (Fig. 2 and Appendix 1, Table S5). In addition, self picking showed a negative correlation with connectivity between the (right dorsal) putamen and the left anterior thalamus/globus pallidus.
Compulsive behaviour severity
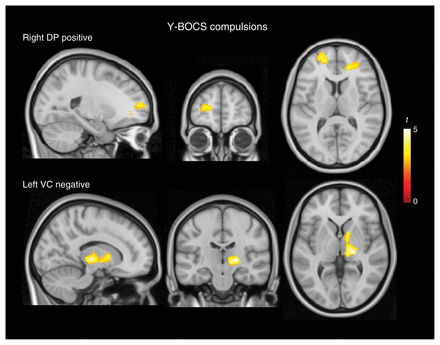
The severity of compulsions measured by the Y-BOCS compulsion scale (Appendix 1, Table S5) was positively correlated with functional connectivity between the (right dorsal) putamen and the anterior prefrontal cortex (frontal pole) and negatively correlated with functional connectivity between the (left ventral) caudate and both the right anterior thalamus and the globus pallidus (Fig. 3 and Appendix 1, Table S5, ).
Correlation analysis results for the severity of compulsions measured by the Yale–Brown Obsessive Compulsive Scale (Y-BOCS). Top: positive correlation of compulsion severity with functional connectivity between the dorsal putamen (DP) and the anterior prefrontal cortex. Bottom: negative correlation of compulsion severity with functional connectivity between the ventral caudate (VC) and both the right anterior thalamus and globus pallidus. The right hemisphere corresponds to the right side of axial and coronal views.
Obsessive–compulsive eating behaviour
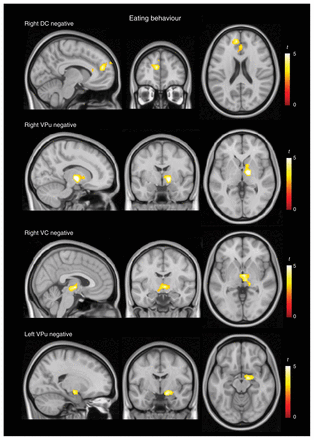
At the cortical level, eating behaviour measurements were negatively correlated with functional connectivity between the caudate nucleus and the anterior extent of the medial frontal cortex and positively correlated with connectivity between the putamen and the somatosensory cortex at the postcentral gyrus. At the subcortical level, eating behaviour was negatively correlated with connectivity between the putamen and the globus pallidus/thalamus and positively correlated with connectivity between both dorsal caudate nuclei and the putamen (Fig. 4 and Appendix 1, Table S6). Significant correlations were also observed beyond the strict cortico–basal ganglia circuits. Eating behaviour ratings were negatively correlated with connectivity between the ventral caudate nucleus and the hypothalamus and with connectivity between the ventral putamen and the amygdala (Fig. 4 and Appendix 1, Table S6).
Correlation analysis results for eating behaviour. Top: negative correlation of eating behaviour scores with functional connectivity between the dorsal caudate (DC) nucleus and the medial frontal cortex and negative correlation with connectivity between the ventral putamen (VPu) and the globus pallidus/thalamus. Bottom: negative correlation of eating behaviour scores with connectivity between the ventral caudate (VC) nucleus and the hypothalamus and with connectivity between the VPu and the amygdala. The right hemisphere corresponds to the right side of axial and coronal views.
As a complementary analysis, we compared patients who had scores of 0 with those who had scores other than 0 for the variables used in the correlations. The results in each case were more robust using the primary correlation approach, which further suggests that a linear association exists between the severity of obsessive–compulsive behaviour and brain functional connectivity.
Discussion
Our study confirms that Prader Willi syndrome is indeed characterized by abnormal functional connectivity of basal ganglia circuits. Demonstrating some overlap with the alterations seen in patients with OCD,10 patients with Prader Willi syndrome showed abnormal connectivity between the prefrontal cortex and basal ganglia and within subcortical structures that correlated with the presence and severity of obsessive–compulsive behaviour. Heightened functional connectivity was additionally identified within the primary sensorimotor cortex–putamen loop, which was strongly associated with self picking. Obsessive–compulsive eating behaviour correlated with the magnitude of functional connectivity both within the basal ganglia loops and between the ventral aspect of the striatum and the hypothalamus and amygdala.
Enhanced activity in ventral prefrontal areas is thought to be a core phenomenon of OCD.35 Former imaging studies of OCD have demonstrated increased functional connectivity between the prefrontal cortex and basal ganglia, further supporting this notion.10,11,36–41 In the present study, increased functional connectivity in basal ganglia loops involving the anterior prefrontal cortex (Fig. 1) and its correlation with the severity of compulsions (Fig. 3) may indicate that Prader Willi syndrome pathophysiologically overlaps, at least in part, with typical OCD. Despite a general consistency, however, our findings also indicate important, distinct features in terms of the striatal and prefrontal cortical territories implicated.10,11,38,41
In patients with Prader Willi syndrome, we identified a relevant increase in functional connectivity between the putamen and primary sensorimotor cortices. Assuming that the primary sensorimotor cortex–basal ganglia loop represents a more direct stimulus–response pathway than the prefrontal–basal ganglia loops, a possible interpretation is that defectively inhibited/impulsive motor responses in patients with Prader Willi syndrome may be underpinned by enhanced functional connectivity within putamen motor circuits. Enhanced functional connectivity in prefrontal loops may instead contribute to compulsive behaviours, such that the response urge is not directly triggered by the stimulus, but is mediated by abnormal activity in the prefrontal cortex. Attributing impulsivity to sensorimotor loop dysfunction and compulsions to prefrontal cortex loop dysfunction is oversimplified, but may be useful as a working hypothesis to explain our present findings.
The observed correlations with self picking behaviours may be of particular relevance to this proposal. Skin picking is very common among patients with Prader Willi syndrome3,42–44 and is often considered a compulsion (included in the Compulsive Behaviour Checklist) that shows moderate correlation with obsessive–compulsive behaviour.42 However, self picking has also been clearly differentiated from the other compulsive behaviour by factor analyses5,27 and may in fact also covary with impulsivity traits.45–47 The functional anatomy of our results was very specific. The presence of self picking was associated with increased functional connectivity between the putamen and a large extension of the sensory cortical homunculus. Interestingly, self picking among patients with Prader Willi syndrome is thought to be maintained or generated by somatosensations.42,48,49 Self picking behaviour was also associated with reduced connectivity between the putamen and the thalamus/globus pallidus. Therefore, we have observed a general correspondence between sensorimotor–putamen loop functional connectivity changes and a behavioural symptom domain showing impulsivity features.
Abnormal functional connectivity within subcortical structures may also help to explain inhibitory control alterations in patients with Prader Willi syndrome. Several subcortical connections within the frontosubcortical loops are inhibitory, including striatum–globus pallidus and globus pallidus–thalamus projections.50 Accordingly, functional connectivity alterations in such pathways could express response disinhibition or facilitation. Because resting fMRI signal in all these structures is strongly, positively correlated (i.e., synchronized activity), it could be argued that subcortical connectivity in these maps better expresses fluctuations of general tonic arousal in the subcortical nuclei, rather than strictly local nucleus to nucleus influences. Our subcortical results may therefore be interpreted as a general pattern of activity uncoupling within the basal ganglia and between the striatum and thalamus, which is generally associated with the broader profile of poorly inhibited behaviour seen in patients with Prader Willi syndrome.
The observed correlations with obsessive–compulsive eating behaviour generally paralleled those observed for other obsessive–compulsive behaviours, involving functional connectivity both between the cortex and basal ganglia and within the basal ganglia. These results further suggest that obsessive eating behaviour is associated with a dysfunctional response control system.
Our results also suggest more complex associations, as the presence and severity of the obsession to eat was additionally associated with abnormal connectivity of the ventral striatum with the hypothalamus and the amygdala. Altered eating behaviour in patients with Prader Willi syndrome is considered to be a failure of satiety51,52 potentially related to hypothalamic dysfunction.53 Our results may provide direct evidence of the abnormal association between very basic limbic structures governing internal homeostasis (i.e., hypothalamus and amygdala) and the ventral frontostriatal system related to motivation, reward and satiety.50,54–56 In other words, the results may suggest how nonsatiated basic drives ultimately favour the generation of obsessions via a deficient inhibition of ventral frontal cortex activity. Reduced ventral frontal inhibition from the hypothalamus is consistent with both its increased response when viewing pictures of food24,25,57,58 and its delayed or reduced satiety responses to oral nutrients,20,21 as reported in prior imaging studies of patients with Prader Willi syndrome.
It is important to mention that basal ganglia–amygdala/hypothalamus functional connectivity did not appear to be abnormal in quantitative terms, as both groups showed similar connectivity measurements. Nevertheless, the correlation findings within the patient group could indicate that hunger and deficient satiety are relevant modulators of the functional connectivity at this level in patients with Prader Willi syndrome. By contrast, activity of this circuit could depend somewhat more on higher-order domains in healthy conditions.
In terms of the clinical relevance of our findings, we would argue that a more comprehensive understanding of the pathophysiology of problematic eating behaviour is of particular importance in Prader Willi syndrome. Satiety is regulated by a complex interaction of hormones and the corresponding brain targets of these hormones are only partially understood. Our findings suggest that functional connectivity alterations in patients with Prader Willi syndrome occur in primary structures linked to the regulation of hunger/satiety. Future imaging investigations will need to address whether these connectivity findings have the potential to represent clinical biomarkers in patients with Prader Willi syndrome.
Limitations
The assessment of obsessive–compulsive behaviour in patients with Prader Willi syndrome is limited owing to the difficulty in evaluating the nature of content of obsessions in patients with reduced insight. Family reports of patients with Prader Willi syndrome clearly indicate that some aspects of the patients’ behaviour is obsessional (e.g., obsessive food searching), but patients generally do not express their intrusive thought content. Another potential limitation in assessing patients with a degree of intellectual disability is the risk of excessive head motion artifact on brain functional connectivity measurements.30 To mitigate this concern, as detailed in the Methods section, we adopted a variety of procedures that have been previously reported to be effective in removing motion effects in such populations. Also, 6-min resting-state fMRI may be considered a relatively short assessment. However, we considered that it would be difficult to keep patients compliant to a longer assessment period without the need for sedation. A final limitation relates to using a 1.5 T imaging system as opposed to a 3 T system with a higher MRI signal.
Conclusion
Patients with Prader Willi syndrome exhibited broad functional connectivity alterations involving prefrontal loop changes associated with obsessive–compulsive behaviour that concurred with other anomalies. Of relevance, enhanced coupling in the primary sensorimotor loop correlated with the most impulsive aspects of the behaviour, and reduced coupling between the ventral striatum and homeostatic limbic structures correlated with the obsession to eat. In the context of frontal–basal ganglia physiology, this study may provide new insights into the nature of obsessive–compulsive behaviour, its boundaries with impulsivity and the causal role of nonsatiated basic drives.
Acknowledgements
This study was supported in part by the Fondo de Investigación Santiaria del Instituto Carlos III (Grant PI-10/00940) and Fundació Parc Taulí (Grants CIR 2010/006 and CIR 2011/004). The Agency of University and Research Funding Management of the Catalonia Government participated in the context of Research Group SGR2014-1673. L. Blanco-Hinojo was supported by the PFIS grant FI10/00387 from the Fondo de Investigación Santiaria del Instituto Carlos III. Dr. B. Harrison was supported by a National Health and Medical Research Council (NHMRC, Australia) Clinical Career Development Fellowship (628509). We thank the Associació Catalana de Síndrome de Prader-Willi, Fundación Síndrome de Prader-Willi and all the patients and their families for agreeing to participate.
Footnotes
Competing interests: None declared.
Contributors: J. Pujol, S. Esteba-Castillo, A. Caixàs, J. Deus and R. Novell-Alsina designed the study. S. Esteba-Castillo, A. Caixàs, M. Bueno, M. Rigla and J. Llorente-Onaindia acquired the data, which J. Pujol, L. Blanco-Hinojo, B. Harrison, D. Macià and R. Novell-Alsina analyzed. J. Pujol, L. Blanco-Hinojo, A. Caixàs and B. Harrison wrote the article, which all authors reviewed and approved for publication.
- Received November 14, 2014.
- Revision received July 22, 2015.
- Accepted September 2, 2015.