Abstract
Background: Data on structural brain alterations in patients with attention-deficit/hyperactivity disorder (ADHD) have been inconsistent. Both ADHD and brain volumes have a strong genetic loading, but whether brain alterations in patients with ADHD are familial has been underexplored. We aimed to detect structural brain alterations in adolescents and young adults with ADHD compared with healthy controls. We examined whether these alterations were also found in their unaffected siblings, using a uniquely large sample.
Methods: We performed voxel-based morphometry analyses on MRI scans of patients with ADHD, their unaffected siblings and typically developing controls. We identified brain areas that differed between participants with ADHD and controls and investigated whether these areas were different in unaffected siblings. Influences of medication use, age, sex and IQ were considered.
Results: Our sample included 307 patients with ADHD, 169 unaffected siblings and 196 typically developing controls (mean age 17.2 [range 8–30] yr). Compared with controls, participants with ADHD had significantly smaller grey matter volume in 5 clusters located in the precentral gyrus, medial and orbitofrontal cortex, and (para)cingulate cortices. Unaffected siblings showed intermediate volumes significantly different from controls in 4 of these clusters (all except the precentral gyrus). Medication use, age, sex and IQ did not have an undue influence on the results.
Limitations: Our sample was heterogeneous, most participants with ADHD were taking medication, and the comparison was cross-sectional.
Conclusion: Brain areas involved in decision making, motivation, cognitive control and motor functioning were smaller in participants with ADHD than in controls. Investigation of unaffected siblings indicated familiality of 4 of the structural brain differences, supporting their potential in molecular genetic analyses in ADHD research.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neuropsychiatric disorder characterized by developmentally inappropriate impulsive, hyperactive and/or inattentive symptoms. 1 Previous MRI studies have reported smaller total brain volumes in participants with ADHD than in controls.2,3 Meta-analyses of regions of interest (ROI) studies have shown significantly smaller volume in total and right cerebral volume, frontal brain areas, the right caudate and cerebellar regions in participants with ADHD than in controls.3 Volumetric ROI studies, however, are restricted to a small number of a priori selected regions, which could give rise to selection bias.
A method that circumvents this potential bias and allows a whole brain, hypothesis-free analysis is voxel-based morphometry (VBM),4 an MRI analysis technique that assesses differences between groups in voxelwise grey matter volume. Three meta-analyses of VBM studies reporting on partly the same samples have investigated the most prominent volumetric differences between participants with ADHD and controls.5–7 The first meta-analysis (114 children with ADHD and 143 controls) reported smaller grey matter volume in the right putamen and globus pallidus in children with ADHD than in controls.5 A more recent meta-analysis (202 children and adolescents and 176 adults with ADHD and 344 controls) confirmed the reduced localized subcortical grey matter findings in the right globus pallidus and putamen and also reported on a smaller right caudate nucleus, whereas larger local grey matter volume was found in the left posterior cingulate cortex.6 The most recent study (175 children and adolescents and 145 adults with ADHD plus 288 controls) found reduced right globus pallidus and putamen volumes in children with ADHD, whereas the adult samples were characterized by anterior cingulate cortex volume reductions.7 However, studies used to generate the meta-analyses showed strong heterogeneity among samples and methods; reliability analysis revealed inconsistency in findings in 50%–75% of the studies.6 Given these limitations, investigating local volume differences within a single large sample has substantial added value over existing meta-analyses.
Mechanisms underlying the association between ADHD and brain volumes are unclear. Both ADHD and brain volumes are known to be subject to genetic and familial influences (i.e., shared genetic and/or shared environmental factors).8,9 This suggests the potential usefulness of brain volumes in the search for ADHD risk genes.10 In this context, unaffected siblings of patients with ADHD are of interest, as they share on average 50% of their genetic material as well as the family environments with the ADHD proband. Consequently, if brain volumes of unaffected siblings are also significantly smaller than those of healthy controls, this would suggest shared familiality between the brain phenotypes and the ADHD phenotype. Two previous studies (n = 90 and n = 60, respectively) reported that unaffected siblings of participants with ADHD had alterations in prefrontal grey matter and occipital grey and white matter as well as inferior frontal gyrus grey matter and inferior fronto-occipital fasciculus white matter intermediate between those of participants with ADHD and controls,11,12 which indicates a shared underlying familial component.
It is important to consider factors that might influence the analysis of case–control differences when studying ADHD. One of these factors is the use of psychostimulant medication in participants with ADHD, with evidence showing that stimulant treatment might normalize specific structural brain abnormalities found in children with ADHD.13 Two of the VBM meta-analyses mentioned previously found that subcortical volume differences between patients with ADHD and controls were smaller when the patients had taken medication.6,7 Further, increasing age was found to be associated with smaller brain volume differences6,7 between participants with ADHD and controls, which is in line with the findings of studies reporting delayed brain maturation in participants with ADHD.2,14 As previous MRI studies of ADHD included mostly children or adults,5–7 the present study adds to the literature by focusing on adolescents and young adults. This allows for an examination of the important time window of the transition from adolescence into early adulthood when studying age effects. It is important to take sex into account, because sex distribution in childhood ADHD is skewed, with boys outnumbering girls;15 brain volume differs between males and females;16 and brain differences in ADHD between males and females have been reported.17 In addition, IQ may influence associations between brain volume and ADHD because, on average, children with ADHD tend to have a somewhat lower IQ than controls and because IQ has been associated with structural brain differences.18
The aim of the present VBM study was 2-fold. First, we sought to investigate which brain areas would show case–control differences using a large sample of adolescents and young adults with ADHD and healthy controls. Second, we sought to examine whether brain volumetric changes found in participants with ADHD relative to controls would also be found in the unaffected siblings of participants with ADHD. We investigated the potentially confounding role of medication use, age, sex and IQ on brain volume differences between participants with ADHD and controls.
Methods
Participants
Participants were recruited from the NeuroIMAGE project, a follow-up (2009–2012) of the Dutch part of the International Multicentre ADHD Genetics (IMAGE) study performed between 2003 and 2006.19 In short, ADHD families with at least 1 child with ADHD and at least 1 biological sibling (regardless of ADHD diagnosis) were recruited, as were control families with at least 1 child and 1 biological sibling with no formal or suspected ADHD diagnosis in any of the first-degree family members. For references and a detailed description of the NeuroIMAGE project and study procedures, see the study by von Rhein and colleagues.20
Inclusion criteria were the same for all participants: age between 8 and 30 years; European Caucasian descent; IQ of 70 or higher; and no diagnosis of autism, epilepsy, general learning difficulties, brain disorders or known genetic disorders. The control families could not have an ADHD diagnosis. Participants were excluded from scanning if they had any contraindication to scanning. The study was approved by the Dutch local medical ethics committees, and after complete description of the study to the participants, we obtained written informed consent.
Diagnostic assessment of ADHD
To confirm the diagnosis of ADHD at the time of enrollment in NeuroIMAGE, all participants were similarly assessed using a combination of a semistructured diagnostic interview and the Conners ADHD questionnaires. Symptom counts were taken from these measurements. A detailed description of diagnostic criteria can be found elsewhere20 and in Appendix 1, available at jpn.ca.
Medication
Information on lifetime use of psychoactive medication (methylphenidate immediate release, methylphenidate extended release, atomoxetine and dexamphetamine) was gathered from pharmacy transcripts and questionnaire reports. We classified individuals who never took medication as medication-naive and compared them to individuals who reported having taken medication during their lifetime.
IQ
We estimated full-scale IQ by combining scores on 2 subtests of the Wechsler Intelligence Scale for Children (WISC; if ≤ 17 yr) or the Wechsler Adult Intelligence Scale III (WAIS-III; if > 17 yr) that show the highest correlations with full-scale IQ score: vocabulary and block design.20
Smoking
Self-reported data on smoking status was collected using questionnaires. We classified smoking status as ever smoked and never smoked.
Imaging data
The MRI scanning was conducted at 2 different locations (Donders Centre for Cognitive Neuroimaging in Nijmegen and VU University Medical Centre in Amsterdam, the Netherlands) using 2 comparable 1.5 T MRI scanners (Sonata/Avanto Siemens) and the same 8-channel head-coil and scan protocols. For each participant we obtained 2 high-resolution T1-weighted magnetization-prepared rapid acquisition with gradient echo (MPRAGE) anatomic scans: 1 before and 1 after a break in a longer scanning session. For participants who had 2 good scans, we averaged the VBM estimates across both scans, thereby improving the signal-to-noise ratio. If only 1 good scan was available, we used a single scan. Each participant’s T1-weigthed scan was normalized to Montreal Neurological Institute (MNI) 152 standard space, bias-field corrected and segmented into grey matter, white matter and cerebrospinal fluid using the unified procedure of the VBM 8.1 toolbox.
Grey matter images were modulated by the nonlinear part of the normalization field and smoothed with an 8 mm full-width at half-maximum Gaussian kernel, providing for an analysis of relative differences in regional grey and white matter volume, corrected for individual brain size (http://dbm.neuro.uni-jena.de/vbm8/VBM8-Manual.pdf). Data analysis was restricted to voxels with grey matter with a probability exceeding 25%, leading to inclusion of a total of 230 135 voxels. Details on MRI acquisition and preparation can be found in Appendix 1.
Statistical analysis
Inferential statistical analyses were conducted using Stata software (StataCorp LP). To investigate case–control differences in brain volumes, we performed multiple regression analyses including the voxelwise grey matter relative volume values as outcome measures and binary ADHD diagnosis (ADHD v. control) as a main effect. Because of evidence for sex effects and linear and quadratic effects of age on brain volumes,21,22 the main effects of age, age2 and sex were included as covariates. Scanner location (Amsterdam v. Nijmegen) was also included as a covariate to account for potential effects of site. As observations were not independent within families (both the ADHD group and the control group included siblings from the same families), we used the “robust cluster” option in Stata, which accounts for the correlation structure of the data in calculating robust standard errors.23 We considered differences to be significant if they survived cluster-mass thresholding with the easythresh option in FSL (www.fmrib.ox.ac.uk/fsl), using an initial cluster forming threshold of z > 3.1. Subsequently, we estimated each cluster’s significance level based on Gaussian random field theory, and those clusters surviving a family-wise error (FWE)–corrected significance threshold of p < 0.05 showing volume differences > 0.1 mL were reported. For comparison we repeated our main analysis in SPM while not taking into account the family relatedness in our sample (Appendix 1, Fig. S1 and Table S1).
Mean voxel values of each cluster showing case–control differences were calculated per individual and entered in a linear regression analysis in Stata, accounting for family relatedness. Familiarity was considered present if unaffected siblings significantly differed in mean voxel values from controls but not from participants with ADHD or if they had brain volumes significantly intermediate to participants with ADHD and controls.
Possible influence of medication use, age, sex, IQ and smoking were carried out as post hoc analyses. As controls and unaffected siblings did not use medication, we stratified the ADHD group by medication use and compared the mean voxel values. To examine age and sex differences, the significant clusters were checked for age effects by including age × diagnosis, age2 × diagnosis and sex × diagnosis interactions in the regression model. This leads to the following regression models: Cluster# = βageXage + βage2Xage2 + βsexXsex + βsiteXsite + βdiagnosisXdiagnosis + βage×diagnosisXage×diagnosis + clustering on family identifier; Cluster# = βageXage + βage2Xage2 + βsexXsex + βsiteXsite + βdiagnosisXdiagnosis + βage2×diagnosisXage2×diagnosis + clustering on family identifier; and Cluster# = βageXage + βage2Xage2 + βsexXsex + βsiteXsite + βdiagnosisXdiagnosis + βsex×diagnosisXsex×diagnosis + clustering on family identifier. To investigate the influence of IQ and smoking, the main analysis was repeated adding IQ and smoking separately as covariates. Because we had a skewed distribution of our groups at the 2 different scan sites, we decided to investigate results separately by site. Finally, as symptom counts were present in the complete sample and correlated with diagnostic status, associations between symptom counts and brain-wide brain volume were investigated in the complete sample. Additionally, analyses on matched subsamples for sex, IQ and scan site are described in Appendix 1.
Results
Sample
Our sample included 672 adolescents and young adults and comprised 307 participants with ADHD, 169 unaffected siblings of participants with ADHD and 196 controls. The ADHD group had a significantly lower IQ than the control group (β = −9.1, t356 = −4.78, p < 0.001) and included significantly more male than female participants (β = −0.19, t501 = −4,39, p < 0.001; Table 1).
Demographic and clinical characteristics of study participants
Differences in grey matter volume between participants with ADHD and controls
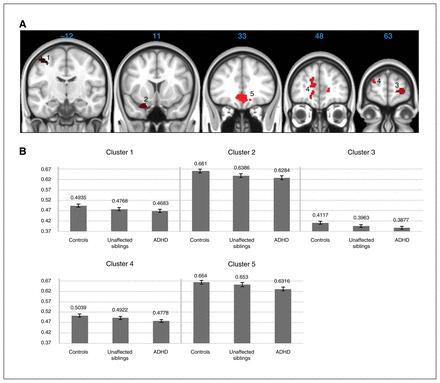
Our whole brain VBM analysis comparing participants with ADHD to controls identified 5 clusters in which participants with ADHD had significantly smaller grey matter volumes than controls. These clusters were located in the precentral gyrus, medial and orbitofrontal cortex, frontal pole and paracingulate and cingulate cortices (Fig. 1, Table 2 and Appendix 1, Fig. S2).
(A) Whole-brain significant clusters for case–control differences. Five clusters were identified: cluster 1 = precentral gyrus; cluster 2 = orbitofrontal cortex; cluster 3 = frontal pole; cluster 4 = paracingulate and cingulate cortices, frontal pole; and cluster 5 = medidal frontal, paracingulate, cingulate and subcallosal cortices. (B) Mean voxel differences for the identified cluster between participants with attention-deficit/hyperactivity disorder (ADHD; n = 307), their unaffected siblings (n = 169) and typically developing controls (n = 196).
Clusters showing whole brain VBM differences between participants with ADHD and controls
Differences between unaffected siblings and participants with ADHD and between unaffected siblings and controls
For all 5 clusters showing significant case–control differences, unaffected siblings showed a pattern of mean voxel volumes intermediate to that of the ADHD and control groups (Fig. 1). In cluster 1 (precentral gyrus), cluster 2 (orbitofrontal cortex) and cluster 3 (frontal pole) the unaffected siblings differed significantly from the controls, but not from participants with ADHD. In cluster 4 (paracingulate and cingulate cortices, frontal pole) all groups significantly differed from each other, whereas for cluster 5 (medial frontal, paracingulate, cingulate and subcallosal cortices) the unaffected siblings differed significantly from the participants with ADHD but not from the controls (Table 3).
Mean voxel values comparisons for participants with ADHD, their unaffected siblings and controls
Possible confounding factors for brain associations
To investigate whether our results were influenced by medication use, age, sex or IQ, we performed a series of sensitivity analyses. No significant differences were observed when the mean voxel volumes of the clusters were compared between the medicated patients (n = 272) and the never-medicated patients (n = 35; Appendix 1, Table S2).
The age × diagnosis, age2 × diagnosis and sex × diagnosis interaction terms added to the regression models investigating the differences in mean voxel values between participants with ADHD and controls were nonsignificant for all 5 clusters (Appendix 1, Tables S3 and S4 and Fig. S3).
When we added IQ as a covariate, we identified an additional significant cluster (171 voxels) in the cuneus for which participants with ADHD showed smaller grey matter volume than controls. The other 4 significant clusters observed in this analysis overlapped with the clusters in the previous analysis (Appendix 1, Fig. S4 and Table S5). The cluster in the precentral area was not significant in this analysis. When we added smoking status as a covariate, all 5 clusters from the main analysis were found again (Appendix 1, Table S6 and Fig. S5).
To investigate whether our results were driven by one of the scan sites our main results are plotted per site in Appendix 1, Table S7. Additionally the results for subsamples carefully matched on sex, IQ and scan site are described in Appendix 1, Table S8. Analyses on subsamples matched for sex, IQ or scan site revealed the same direction of the neural effects for all peak voxels.
Differences in grey matter volume with increasing number of ADHD symptoms
An additional dimensional analysis of the number of ADHD symptoms in the complete sample (ADHD, unaffected siblings and controls) at the whole brain level identified 7 clusters in which more ADHD symptoms were associated with significantly smaller grey matter volumes. These clusters overlapped with our main analyses and were located in the medial and orbitofrontal cortices, frontal pole, paracingulate and cingulate cortices. The precentral gyrus cluster reported in our main analyses did not show up in the symptom count analyses. We found additional clusters in the fusiform gyrus, precuneous and frontal operculum (Appendix 1, Table S9).
Discussion
The present study localized alterations of grey matter volume in participants with ADHD and examined their underlying familial components in a uniquely large sample of adolescents and young adults with ADHD, their unaffected siblings and controls. Compared with controls, participants with ADHD had smaller grey matter volume in 5 clusters, including the left precentral gyrus, medial and left orbitofrontal cortices, frontal pole and paracingulate and cingulate cortices. Unaffected siblings exhibited smaller grey matter volumes than controls in 4 clusters and showed an overall intermediate pattern compared with that of participants with ADHD and controls, indicating familial underpinnings. We could not detect any effect of medication use or evidence that age, sex or IQ had a significant impact on our findings.
Findings in the precentral gyrus, which is linked to motor control, and the prefrontal cortex, which is the target of ADHD medication,24 are in line with and extend the findings of previous VBM studies,25,26 cortical thickness studies27 and region of interest studies3 on structural differences in the precentral gyrus and prefrontal cortex in individuals with ADHD. An important role of abnormalities of the prefrontal cortex and its connections in ADHD is indicated.28 However, findings in the prefrontal cortex have been inconsistent and are not supported by meta-analyses.5–7 A possible contributing factor is the heterogeneity in age among studies, as development of the prefrontal cortex is late and protracted.21
Subtyping of ADHD has been proposed based on dorsal–frontostriatal, orbitofrontal–striatal and fronto–cerebellar circuits involved in cognitive control, reward and motivation, and timing and building temporal expectations, respectively.29,30 While meta-analyses of VBM studies have shown corresponding subcortical regions to be altered in individuals with ADHD5–7 and while cerebellar volume differences have been reported,3,31 our study did not reveal structural differences in these regions. Possible explanations for this might be the adolescent age range of our sample and the previously reported normalization of differences in caudate nucleus volume with age where other brain abnormalities between participants with ADHD and controls persisted during development.2 Also, medication use might have contributed to the absence of findings for subcortical structures.6 However, a meta-analysis on manual tracing studies of subcortical structures did find mean differences in caudate nucleus volume even without findings in VBM.7 A volumetric study segmenting the complete volume of the caudate nucleus and the putamen in the present sample also showed differences in both to be present.32 Such findings could indicate that the differences in caudate nucleus and putamen volume in individuals with ADHD are dispersed as opposed to localized to the very same cluster of voxels and hence not easily detected with VBM.
To date, brain volumetric studies in individuals with ADHD have only rarely included unaffected siblings.11,12 Our findings in a larger sample including both adolescents and adults provide important insight into the familial underpinnings of structural brain differences in individuals with ADHD. Familial components can in theory be due to shared genetic and/or shared environmental factors. Since there is strong evidence for familial components of ADHD to be largely genetic,9,33 we hypothesize the familial effects in the reported brain areas to be mainly driven by genetic factors. Of relevance, a twin study investigating voxel-based brain volume in high- and low–ADHD risk concordant or discordant twin pairs reported genetically mediated risk for ADHD in the medial orbitofrontal areas;34 this finding overlaps with our results. If confirmed, the identified areas may prove useful in molecular genetic research on ADHD.
We investigated whether medication use, age, sex or IQ influenced our results. Null findings for medication use might have been due to power limitations, as the majority of our ADHD group had used medication. This fact might also have influenced our results (i.e., based on meta-analytic results, smaller case–control differences can be expected with medication use6). The findings from our analyses show developmentally stable smaller grey matter volume in frontal brain areas and thereby suggest a deviant development of these brain areas as supposed to a delayed development in participants with ADHD. Findings from our analyses in a rather large sample of participants aged 8–30 years indicate that individuals with ADHD do not “catch up” their delay in brain volume differences in the reported clusters, but rather remain different from healthy controls. As developmental delay has been suggested and reported in prefrontal areas of the brain in individuals with ADHD,14 longitudinal studies are warranted to investigate whether these changes remain throughout adulthood. Although we had a sex distribution imbalance in our sample, post hoc analyses did not indicate that our results are driven by sex-specific differences.
When including IQ in the analysis, the precentral gyrus finding was no longer significant, and an additional region in the cuneus reached significance. To our knowledge, volumetric differences in the cuneus have not been reported previously in ADHD samples; however, an association between inattentive symptoms and resting state activitity in the cuneus have recently been shown.35 The cuneus finding was subthreshold in our main analysis and also became apparent in the analysis that was not corrected for family structure (Appendix 1). There is an active debate whether IQ needs to be included as a covariate in investigations of ADHD.36 Individuals with ADHD have an IQ that is generally about 10 points lower than in healthy participants,37 which could argue in favour of including IQ as a covariate. However, IQ might share meaningful variance with ADHD,19 at least partially, and therefore covarying for IQ might lead to an overcorrection. In the present study, both analyses are provided. The overlapping results of our main analyses and the dimensional analysis on symptom counts are in line with the view of ADHD as an extreme on a continuum of behaviour.38 By using dimensional data, spanning the range from normal to abnormal, knowledge can be gained on how constructs are related to disorders. For all reported clusters an increase in symptom counts was related to smaller volume, which is in line with the findings of previous studies reporting reduced brain volume with increasing ADHD severity.39,40
Limitations
Our findings should be viewed in light of strengths and limitations. We improved upon previous studies by including a large sample, including unaffected siblings, and both adolescents and young adults. Another strength is the use of a whole brain, hypothesis-free approach. As the present study consisted of a large naturalistic group of participants with ADHD and controls, differences in IQ and sex were present in our sample, which is frequently the case in studies of ADHD.15,19 Similarly, most of the participants with ADHD had used medication. An alternative approach to the sensitivity analyses presented would have been to specify groups before analysis (e.g., by investigating only medication-naive participants with ADHD matched to controls by age, sex, IQ and psychiatric comorbidity), 31 but the trade-off would have been a smaller sample size consisting of atypical ADHD cases. Head movement is an important topic in ADHD research. Hence, we performed several steps to minimize movement during scanning and carefully assessed data quality for any potential movement-related artifacts.20 Additionally, we collected 2 structural MRI scans, quality-controlled all of them and excluded scans with excessive motion. For participants with 2 good scans, estimated volumes were averaged across scans to improve signal-to-noise ratio. As 2 scan sites were used for data collection, we included scan site as a covariate in our main analyses; however, this linear correction might not be sufficient. Post hoc analyses matched by scan site and replicating findings separately within each scan site suggest that our results were not driven by scan site–specific differences.
Conclusion
Several lines of future research follow from the present study. First, longitudinal studies are warranted to properly investigate developmental trajectories of brain differences in individuals with ADHD. Second, molecular genetic analyses on brain phenotypes with familial underpinnings can aid the search for and understanding of genetic risk factors for ADHD.
The present VBM analysis of MRI scans in a large sample of participants with ADHD, unaffected siblings and controls found that ADHD was associated with smaller grey matter volume in frontal and precentral areas of the cortex, involving decision making, executive functioning and motor functioning areas. The identified clusters are potentially linked to familial risk for ADHD.
Acknowledgements
The authors thank all the parents, teachers and children who participated in the study. Funding support for the IMAGE project was provided by the National Institutes of Health grants R01MH62873 and R01MH081803. The NeuroIMAGE project was supported by NWO Large Investment Grant 1750102007010, ZonMW Grant 60-60600-97-193 and NWO Brain and Cognition grant 433-09-242 to J. Buitelaar, funding from the European Community‘s Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 305697, the EU FP7 grant TACTICS (grantnr 278948), and grants from Radboud university medical center, University Medical Center Groningen and Accare, and VU University Amsterdam. The research described here also received funding from the National Institutes of Health (NIH) Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centers of Excellence.
Footnotes
↵* These authors contributed equally to this work.
Competing interests: J. Oosterlaan has received an unrestricted grant from Shire outside the submitted work. P. Hoekstra has received honoraria from Eli Lilly and Shire and an unrestricted research grant from Shire outside the submitted work. He also declares serving on the advisory board of Shire. J. Buitelaar declares consulting for, serving on the advisory board of and receiving speaker fees from Janssen Cilag BV, Eli Lilly, Bristol-Myer Squibb, Shering Plough, UCB, Shire, Novartis and Servier. No other competing interests declared.
Contributors: J. Bralten, C. Greven, B. Franke, M. Mennes, M. Zwiers, C. Hartman, J. Oosterlaan, D. Heslenfeld, A. Arias-Vasquez and J. Buitelaar designed the study. N. Rommelse was one of the people who acquired the data, which J. Bralten, C. Greven, B. Franke, M. Mennes, M. Zwiers, D. van der Meer, L. O’Dwyer, P. Hoekstra and J. Buitelaar analyzed. J. Bralten, C. Greven, M. Mennes, N. Rommelse and A. Arias-Vasquez wrote the article, which all authors reviewed and approved for publication.
- Received December 16, 2014.
- Revision received May 28, 2015.
- Accepted September 2, 2015.