Abstract
Background: Altered levels of urocortin 1 (Ucn1) in the centrally projecting Edinger–Westphal nucleus (EWcp) of depressed suicide attempters or completers mediate the brain’s response to stress, while the mechanism regulating Ucn1 expression is unknown. We tested the hypothesis that microRNAs (miRNAs), which are vital fine-tuners of gene expression during the brain’s response to stress, have the capacity to modulate Ucn1 expression.
Methods: Computational analysis revealed that the Ucn1 3′ untranslated region contained a conserved binding site for miR-326. We examined miR-326 and Ucn1 levels in the EWcp of depressed suicide completers. In addition, we evaluated miR-326 and Ucn1 levels in the serum and the EWcp of a chronic variable mild stress (CVMS) rat model of behavioural despair and after recovery from CVMS, respectively. Gain and loss of miR-326 function experiments examined the regulation of Ucn1 by this miRNA in cultured midbrain neurons.
Results: We found reduced miR-326 levels concomitant with elevated Ucn1 levels in the EWcp of depressed suicide completers as well as in the EWcp of CVMS rats. In CVMS rats fully recovered from stress, both serum and EWcp miR-326 levels rebounded to nonstressed levels. While downregulation of miR-326 levels in primary midbrain neurons enhanced Ucn1 expression levels, miR-326 overexpression selectively reduced the levels of this neuropeptide.
Limitations: This study lacked experiments showing that in vivo alteration of miR-326 levels alleviate depression-like behaviours. We show only correlative data for miR-325 and cocaine- and amphetamine-regulated transcript levels in the EWcp.
Conclusion: We identified miR-326 dysregulation in depressed suicide completers and characterized this miRNA as an upstream regulator of the Ucn1 neuropeptide expression in midbrain neurons.
Introduction
Suicide ranks among the leading causes of death globally and takes a heavy emotional and public health toll on most societies.1,2 The current understanding of suicidal behaviour is based on a stress-vulnerability model. A large body of evidence supports the involvement of neuropeptides in stress-mediated disorders, such as major depressive disorder (MDD) and suicidality.3–6 In most cases, however, the regulatory mechanisms driving the expression changes in these neuropeptides have remained elusive. For a better understanding of the stress response and future intervention strategies, the mechanisms modulating the expression levels of neuropeptides require elucidation.
Previous studies have identified microRNAs (miRNAs) as indispensable gene regulators that are expressed in both the developing and adult mammalian brain.7,8 MicroRNAs are noncoding gene transcripts that regulate gene expression by binding to the 3′-untranslated region (3′-UTR) of target messenger RNAs (mRNAs), thereby inhibiting protein synthesis or causing mRNA degradation.9,10 Hence, an enticing pathway for regulation of the neuronal stress response is the direct targeting of stress-sensitive neuropeptides by miRNAs.11,12 To date, however, a direct regulation of neuropeptides by miRNAs has not been demonstrated.
Recent studies suggest that miRNAs have the capacity to modulate the neuronal stress response.13 For example, acute stress alters miR-183 and miR-134 levels in the amygdala,14 whereas miR-144 and miR-16 levels are altered in the blood in a naturalistic stress situation, such as academic examinations.15
At present, it is challenging to model human suicidal behaviour in rodents. However, risk factors for suicidal behaviour (e.g., aggression, impulsivity, irritability, behavioural despair) can be modelled to explore the mechanisms underlying the association between stress and suicide.16 In line with these efforts, we used the chronic variable mild stress (CVMS) paradigm to induce stress vulnerability and used the forced swim test (FST) to assess stress-induced behavioural despair in rats.16 Additionally, postmortem brain samples of the rostroventral midbrain of depressed suicide completers were examined for altered miRNA levels in stress response. Using this approach, we tested the hypothesis that miRNAs would directly modulate stress-induced neuropeptide expression, resulting in the identification of selective miRNAs as upstream modulators of these neuropeptides. This postulate was tested in a stress-sensitive model system, the midbrain urocortin 1 (Ucn1) neuronal system. Ucn1 neurons in the rostroventral midbrain, within the centrally projecting Edinger–Westphal nucleus (EWcp) are sensitive to stress:17–21 mice lacking Ucn1 reveal anxiogenic behaviour,22 while depressed suicide completers have upregulated midbrain Ucn1 mRNA expression compared with naturally deceased control individuals.23
Here, we report that the brain-enriched miR-326 acts as an upstream regulator of Ucn1 during a neuronal stress response. These results are also, to our knowledge, the first to identify a miRNA-mediated neuropeptide expression pathway in stress and associated suicide.
Methods
Animals
Thirty-six male albino Wistar-R Amsterdam rats were bred in house at the Animal Facility, Department of Anatomy, Pécs, Hungary. Animals were group-housed in standard plastic cages (40 × 25 × 20 cm), in a temperature- and humidity-controlled environment. The animals were kept on a 12-hour light–dark cycle (lights on at 6 am, 200 lux light intensity). Feeding was ad libitum. For the CVMS experiments, rats (12–14 wk old) were divided into 4 groups (n = 8 animals per group): a CVMS group, a CVMS recovery group and 2 control groups. The CVMS and the CVMS recovery groups were exposed to a CVMS protocol for 14 days (for a detailed protocol see the study by Willner and colleagues24). Rats in the CVMS recovery group were left to rest and were not disturbed for 3 months after stress. Control animals were not exposed to a stress protocol. One control group of rats was sacrificed together with rats exposed to CVMS. Rats assigned to the other control group were sacrificed later with rats in the CVMS recovery group in order to exclude possible effects of aging on measured indices. Animal body weights were measured throughout the experimental period. Following CVMS, all animals were subjected to the FST to assess depression-like behaviour. Rats were decapitated 1–2 min after the FST, and 1.0 mL trunk blood was collected. We measured plasma corticosterone levels using a corticosterone radioimmunoassay as described previously.25 All groups were sacrificed directly after completion of the stress paradigm and subsequent measurements. Brains were removed and placed on dry ice. All procedures were conducted in accordance with the declaration of Helsinki and the animal use guidelines approved by the Medical Faculty Advisory Committee for Animal Resources of Pécs University based on Act XXVIII of 1998 on the protection and sparing of animals.
Rats used for embryonic midbrain isolation were individually housed in standard plastic cages under normal conditions with a 12-hour light–dark cycle (as described earlier). Feeding was ad libitum. Operations were performed at embryonic day 18. Prior to operation, pregnant rats were anesthetized using fluoroethane and sacrificed by cervical dislocation. We retrieved 4–5 rat embryos and euthanized them. Decapitation was performed with surgical scissors. Embryonic brains were transferred onto a plastic dish on ice for the preparation of midbrain neurons as described below. All experiments were approved by the Committee for Animal Experiments of the Radboud University Nijmegen Medical Centre (approval code RU-DEC2010–118), Nijmegen, The Netherlands.
Cell culture
Rat neuroblastoma B35 cells were used for the luciferase gene activity assays and were obtained from the American Type Culture Collection. Cells were cultured in Dulbecco’s Modified Eagle Medium high glucose (4.5 g/L) supplemented with pyruvate (10 mg/mL), penicillin/streptomycin antibiotics (20 μg/mL) and 10% fetal calf serum, and cultures were maintained at 37°C (5% CO2).
Primary cultures of midbrain neurons were prepared from embryonic day 18 rats. Isolated midbrain sections (dissected as described in the study by Son and colleagues26) were transferred to ice-cold Hank’s Balanced Salt Solution (HBSS; Life Technologies), supplemented with 500 units of penicillin and 500 μg of streptomycin directly after isolation. Midbrains were then washed twice with 12 mL of HBSS and trypsinized in 4 mL of HBSS with 0.5% trypsin. After 15 min of incubation at 37°C, trypsin was removed and midbrains were washed 3 times with ice-cold HBSS. After washing, midbrain cells were dissociated in 4 mL of seeding medium, consisting of neurobasal medium (Life Technologies), 10% fetal calf serum (FCS) and 2 mmol/L of GlutaMAX (Life Technologies) by pipetting gently up and down with a glass pipet, followed by pipetting with a fire-polished glass pipet. After dissociation, cells were plated into glass cover slips in a 24-well plate at a density of 600 000 cells per glass slide. Glass slides were pretreated for 72 h with nitric acid and 72 h with deionized water and coated with 1:10 poly-d-lysine (0.1 mg/mL) in phosphate-buffered saline (PBS) for 24 h. Glass cover slides were washed 4 times with PBS and incubated in seeding medium. Finally, 4 h after plating, seeding medium was replaced by culture medium consisting of neurobasal medium supplemented with B27 (Invitrogen) and 2 mmol/L of GlutaMAX.
Reverse transcription and quantitative real-time polymerase chain reaction
We completed the isolation of total RNA from postmortem human brain samples as previously described.5 After rat EWcp dissection,6 total RNA was isolated from this brain region using TRIzol (Life Technologies) according to the manufacturer’s protocol. We isolated miRNA from human brain samples and serum and brain miRNAs from CVMS rats using the mirVana-PARIS kit (Life Technologies) according to the manufacturer’s protocol. The purity of all isolated RNA samples was determined using agarose gel electrophoresis and UV-spectrophotometric analysis. The mean and standard deviation of the 260/280 nm ratios was 2.0 ± 0.05. We further examined the integrity of the isolated RNA using the BioAnalyzer (Agilent). We synthesized complementary DNA (cDNA) from 0.5–1 μg of RNA according to the protocol provided with the revertAid First Strand cDNA Synthesis Kit (Fermentas). We performed cDNA synthesis for miRNA expression detection using the miScript Reverse Transcription Kit (Qiagen) as previously described.7 Semiquantitative real-time polymerase chain reaction (qRT-PCR) was performed with 1/10 diluted cDNA using the Maxima SYBR green kit (Fermentas) according to the manufacturer’s protocol for detection of mRNA using primers designed with vector NTI software. Relative mature miRNA expression levels were analyzed using the miScript SYBR Green PCR Kit (Qiagen) according to the manufacturer’s protocol. We performed qRT-PCR using a Corbett Rotor-gene 6000 Real-time PCR machine (Qiagen). Relative gene expression values were calculated using the delta Ct method.8 The relative expression levels of the housekeeping genes small nuclear RNA (snRNA) U6 and β-actin were used to normalize for miRNAs, cocaine- and amphetamine-regulated transcript (CART), and Ucn1 levels. The housekeeping genes were stably expressed in all samples examined, suggesting that they could serve as endogenous references for the quantifications of miRNAs and coding transcripts using qPCR assays. A list of the primers used is available in Appendix 1, Table S1, available at jpn.ca).
In situ hybridization analyses
Four male rats were used for in situ hybridization (ISH) and immunohistochemistry (IHC) studies. We performed ISH detection of miR-326 on free-floating rat midbrain sections using DIG-labelled locked nucleic acid (LNA) probes (Exiqon) according to the manufacturer’s protocol. Rostroventral midbrain sections containing the EWcp of 4 control rats were fixated overnight in 4% paraformaldehyde (PFA) before rinsing in PBS. After rinsing, sections were incubated in proteinase K medium containing 0.1 mg of proteinase K (Invitrogen), 0.1 M of Tris HCl, and 0.05 M of ethylenediaminetetraacetic acid (EDTA) for 10 min at 37°C. After deproteinization, sections were rinsed in 0.1 M of triethanolamine buffer and incubated for 10 min in 0.25% acetic acid anhydride with 0.1 M of triethanolamine. Sections were then rinsed in 2× saline sodium citrate (SSC) buffer followed by overnight hybridization to a nontargeting Scramble-miR (Exiqon) control and rat miR-326 predesigned LNA probes (Exiqon) at 58°C. Following hybridization, sections were washed in 2× SSC buffer and incubated in ribonuclease (RNase) medium (Roche) for 30 min at 37°C. After successively rinsing sections in 2×, 1× and 0.5× SSC buffer, sections were incubated in 0.1× SSC for 30 min at 58°C. Sections were subsequently preincubated in Tris buffer containing 0.1 M of Tris HCl and 0.15 M of NaCl for 1 h, followed by incubation in 100 μL of Sheep-anti-DIG-AP at a 1:5000 dilution containing 0.5% blocking agent for 3 h. Subsequently, sections were washed in Tris buffer containing 0.1 M of Tris HCl and 0.15 M of NaCl and in Tris buffer containing 0.1 M of Tris HCl, 0.05 M of MgCl2, and 0.15 M of NaCl. After washing, sections were incubated overnight in NBT/BCIP medium containing 2.4 mg of levamisole (Sigma Aldrich) and 175 μL of NBT/BCIP (Roche), followed by rinsing in Tris buffer containing 0.1 M of Tris HCl and 0.01 M of EDTA. Sections were mounted on gelatin-coated glass slides and cover-slipped with Kaiser’s solution. Photomicrographs were taken using a Leica DMRBE microscope connected to a CD 500 digital camera (Leica Microsystems).
Immunohistochemical analysis
Immunohistochemical detection of Ucn1 peptide was performed on free-floating rat midbrain sections as described previously.27,28 Sections were washed 3 times for 10 min in 0.1 M of PBS followed by incubation in 0.1% Triton X-100 in PBS for 30 min. Next, sections were incubated overnight at 4°C with the primary antibody Ucn1 anti-rabbit (gift from W.W. Vale, Salk Institute) at a 1:5000 dilution, with 2% normal donkey serum (Jackson Immunoresearch) in PBS. After a wash in PBS, sections were incubated with biotin-SP conjugated donkey anti-rabbit immunoglobulin (IgG; #771–065–152; Jackson Immunoresearch) diluted at 1:200 in PBS containing 2% normal goat serum for 2 h at 20°C. Sections were rinsed in cold PBS, incubated in avidin–biotin complex (Vectastain Elite ABC Kit, Vector Laboratories) for 1 h at 20°C and rinsed with PBS (3 times for 10 min) and with Tris buffer (pH 7.6) for 10 min. Finally, they were treated successively with 0.05% diaminobenzidine (DAB) in the Tris buffer for 10 min with 0.02% DAB in this buffer for 10 min and with 0.00003% H2O2 for about 10 min. After 3 consecutive 10-min washes in PBS, sections were mounted on gelatin-coated glass slides, air-dried, treated with xylene twice for 10 min and cover-slipped with DePex. Photomicrographs were taken using a Leica DMRBE microscope connected to a CD 500 digital camera (Leica Microsystems).
Postmortem human brain samples
Brains were obtained at autopsy from the Department of Forensic Medicine of Semmelweis University, Budapest, Hungary. Brains were microdissected and tissue samples stored in the Human Brain Tissue Bank, Budapest. All sample donors were white men from Hungary (Budapest region).23 Brains from suicide completers with MDD (5 men) and from controls (8 men) were studied (Appendix 1, Table S1). The average age of male controls and suicide completers was 51.8 ± 6.5 years and 40.0 ± 8.7 years, respectively. The average postmortem delays were 2.7 ± 1.6 h for male controls and 4.3 ± 1.6 h for male suicide completers. We found a significant difference between the average ages of the sample donors (p = 0.033), but no difference between the postmortem delays in obtaining the brain samples (p = 0.09) of male controls and suicide completers. The medical, psychiatric and drug history of all suicide completers was obtained through chart review coupled with interviews with the attending physician(s)/psychiatrist(s) and family members.
Psychiatric evaluation for every suicide completer was made using psychological autopsies. Briefly, DSM-IV diagnoses were established by a panel of clinicians based on information obtained by means of proxy-based interviews complemented by medical and coroner records. Individuals included in this investigation consisted of suicide completers with recurrent MDD (nonbipolar depression). Suicide completers with a history of schizophrenia, epilepsy, or alcohol or other drug abuse were excluded. All suicide completers died by hanging. Some of them had previously been treated for major depression, but details on the dose of medication or level of compliance with therapy could not be reliably obtained in each case (Appendix 1, Table S1). Nevertheless, psychological autopsy and medical records indicated that none of the individuals included in the study had received antidepressant medication for at least 2 months before death. In addition, toxicological tests on blood samples did not reveal the presence of drugs or alcohol in individuals who died by hanging (Appendix 1, Table S1). All controls died suddenly of acute respiratory failure or myocardial infarction (Appendix 1, Table S1). Psychological autopsy based on medical records confirmed the absence of psychiatric illness, antipsychotic medications and alcohol or drug abuse during the previous 10 years. In addition, systematic neuropathological analysis (histopathology) did not show neurodegenerative disorder for any individuals (Appendix 1, Table S1). Harvesting of tissues was approved by the local ethics committee of Semmelweis University, and written informed consent had been obtained from next of kin for use of brain material and clinical data. Samples were collected as described previously.23
Luciferase gene activity assay
Forty-eight hours after transfection, B35 cells were lysed and processed for luciferase luminescence measurements. For detection of luciferase activity, we used the Dual-Glo luciferase assay system (Promega) as previously described.29 Briefly, the acquired amount of Dual-Glo reagent was added to the cell medium, enabling cell lysis and subsequent detection of firefly luminescence in a luminometer. Normalization of the samples was performed by adding the Dual-Glo Stop & Glo reagent, enabling the detection of renilla luminescence. The luciferase activity in relative light units (RLU) was subsequently calculated.
Statistical analysis
Quantitative data are presented as means ± standard errors of the mean (SEM). For the analysis of the postmortem brain data, we used the nonparametric Wilcoxon tests with Bonferroni correction. For rodent data, we used 1-way analysis of variance (ANOVA) with Bonferroni multiple comparison tests to analyze significant differences among multiple groups. We considered results to be significant at p < 0.05. To test the association between paired miRNA–mRNA expression levels within groups, we calculated the Pearson correlation coefficients and p values using GraphPad software.
Results
Identification of stress-modulated EWcp neuropeptides as putative miRNA targets
We examined whether miRNA may act to modulate the levels of the stress-mediated neuropeptides CART and Ucn1 in the EWcp. We chose these 2 neuropeptides because the central stress regulatory pathways particularly use Ucn1 and CART, which have been shown to act together in response to acute stress.27 To assess the potential regulatory impact of miRNAs, we screened different miRNA target databases for miRNAs able to bind to the 3′-UTRs of stress-modulated neuropeptides. This search revealed several miRNA-binding sites in transcripts encoding Ucn1 and CART known to be upregulated in the EWcp in response to stress in humans and rodents.20,30 As shown in Table 1, the TargetScan algorithm31 revealed that the miR-326 binding sites in the 3′-UTR of Ucn1 mRNA were highly conserved across humans, rats and mice, while computational analysis identified that the 7-mer miR-326 binding sequences within the 3′-UTR of CART mRNA were not conserved across these species. Moreover, computational analysis predicted miR-325 only as a putative upstream regulator of CART in mice and rats.
Ucn1 mRNA is a putative miR-326 target
MicroRNA-326 is downregulated in depressed men
Previously, we demonstrated that the expression of UCN1 and CART in rostroventral midbrain punches of postmortem brain samples of drug free, depressed men who committed suicide was markedly upregulated.23,30 To assess whether the expression of the in silico–predicted miR-325 and miR-326 correlated with the previously observed elevated levels of these neuropeptides, we examined UCN1 miR-325 and miR-326 levels in the EWcp of depressed men who committed suicide. When comparing their levels with those of controls, we detected a 4.43-fold increase in UCN1 mRNA levels and a 4.75-fold reduction in miR-326 levels (Fig. 1A and B). In contrast, miR-325 levels were comparable between depressed suicide completers and controls. Relative mean miR-325 levels in controls and suicide completers were 0.15 ± 0.014 and 0.135 ± 0.028, respectively.
Urocortin 1 (UCN1) and microRNA (miR)-326 levels are altered in depressed suicide completers. (A) Quantitative real-time polymerase chain reaction (qRT-PCR)–based quantification of relative UCN1 messenger RNA levels in matched controls (white bar) and depressed suicide completers (black bar). (B) Quantitative RT-PCR-based quantification of relative miR-326 levels in matched controls and depressed suicide completers reveals decreased miR-326 expression levels in suicide completers. The relative expression levels were normalized to the expression of the housekeeping genes small nuclear RNA U6 and β-actin. *p < 0.05, **p < 0.01, 1-way analysis of variance.
Micro-RNA-326 is upregulated and Ucn1 mRNA is downregulated in rats after CVMS
To assess the effect of stress on miR-325 and miR-326 levels in the EWcp, expression levels of these miRNAs along with the mRNA levels of their putative downstream neuropeptide target mRNAs were investigated in the CVMS rat model of behavioural despair.32,33 The CVMS-exposed male rats displayed behavioural despair in the FST, reflected in an approximate 45% increased immobility (p < 0.001) and an approximate 60% (p < 0.001) decreased struggling (Appendix 1, Fig. S1).
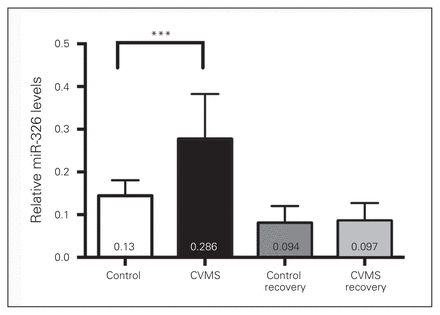
In addition, sustained activation of hypothalamic–pituitary–adrenal (HPA) axis is a characteristic hallmark of depression.4,34,35 This phenomenon is tested in animal models by assessing various physiologic parameters, such as tissue weight and blood levels of corticosterone. Rats exposed to CVMS showed significant decrease in body weight gain (p < 0.014) and an approximate 30% decrease in thymus weight (p < 0.001), as well as a trend toward elevated blood corticosterone levels (p = 0.06) and decrease in adrenal weight (p = 0.07; Appendix 1, Fig. S1). A comparative qRT-PCR experiment on total RNA extracted from micropunches of EWcp confirmed previous findings,20,36 namely that levels of CART and Ucn1 mRNAs were upregulated in CVMS rats compared with nonstressed controls (Fig. 2A). While miR-325 levels were not significantly reduced in the CVMS rats, the relative abundance of EWcp miR-326 levels in the CVMS condition were significantly downregulated compared with nonstressed controls. This resembles the observation for this miRNA in the postmortem EWcp of suicide completers (Fig. 2A). Interestingly, expression of miR-326 rebounded to basal levels in the EWcp of CVMS animals left to recover for 3 weeks (Fig. 2B). The upregulation of Ucn1 and CART mRNAs and downregulation of miR-326 exhibited significantly negative correlations (Fig. 2C and D), suggesting that upregulation of neuropeptide transcript levels is concomitant with a downregulation of miR-326 in the EWcp of rats. The CART mRNA levels showed a positive correlation with Ucn1 mRNA levels (Fig. 2E). No significant negative correlation was computed between miR-325 and CART mRNA levels (data not shown). To visualize the presence of miR-326 in EWcp, we conducted ISH experiments in the rat brain. We found that miR-326 was clearly visible in EWcp cells and co-localized with puncta depicting immunostained Ucn1 (Fig. 2F).
Downregulation of microRNA (miR)-326 and miR-325 expression and upregulation of urocortin 1 (Ucn1) and cocaine- and amphetamine-regulated transcript (CART) messenger RNA levels in chronic variable mild stress (CVMS)–exposed rats. (A) Quantitative real-time polymerase chain reaction (qRT-PCR)–based quantification of relative Ucn1 and CART mRNAs and miR-326 and miR-325 expression levels in the Edinger–Westphal nucleus (EWcp) revealed increased expression for both Ucn1 and CART mRNA in CVMS-exposed rats (black bars) compared with control rats (white bars). The miR-326 levels decreased in CVMS-exposed compared with control rats. Bars indicate means ± standard errors of the mean (SEM) of 8 animals per group. The relative expression levels were normalized to the expression of small nuclear RNA (snRNA) U6 and β-actin. The control group is set at 100%. *p < 0.05, **p < 0.01, ***p < 0.001, 1-way analysis of variance. (B) The miR-326 and miR-325 expression in the EWcp of control and recovered CVMS-treated rats. Quantitative RT-PCR-based quantification of miR-326 and miR-325 expression levels in the EWcp of stress-recovered rats suggest that the levels of these miRNAs are returning to basal levels. Bars indicate means ± SEM of 8 animals per group. The relative expression levels were normalized to the expression of the housekeeping genes snRNA U6 and β-actin. (C) The Ucn1 mRNA and miR-326 levels exhibited a negative expression correlation as represented by Pearson correlation analysis. (D) CART mRNA and miR-326 expression levels exhibited a negative correlation as represented by Pearson correlation analysis within different groups. (E) CART mRNA levels showed a positive correlation with Ucn1 mRNA levels. (F) MicroRNA-326 colocalizes with Ucn1 neuropeptides in the same neurons of the EWcp in rats. The left and middle panels are photomicrographs of (left) mirror-adjacent sections depicting Unc1 immunoreactivity and (middle) expression of miR-326. The arrows point to cells colocalizing Ucn1 and miR-326. Asterisks depict corresponding blood vessels as orientation points in the adjacent slides. (Right) In situ hybridization using a scrambled miR control probe in the EWcp. Aq = cerebral aqueduct. Scale bar = 25 μm.
To determine whether miR-326 levels could be detected in serum and change as a consequence of stress, we isolated serum from CVMS rats, CVMS-recovered rats and nonstressed controls. The qPCR analysis of total RNA isolated from the serum of these rats revealed that miR-326 levels were higher in CVMS rats than in nonstressed control rats. No Ucn1 mRNA was detected in the rat serum. Similar to the previous observation in the EWcp of stress-recovered rats, serum miR-326 levels rebounded to control levels upon stress recovery (Fig. 3).
Elevation of microRNA (miR)-326 levels in the chronic variable mild stress (CVMS) rat serum. The expression levels of miR-326 in control, CVMS and CVMS-recovered rat serums were normalized to both small nuclear RNA U6 and β-actin. Bars indicate means ± standard errors of the mean of 8 animals per group. ***p < 0.001, 1-way analysis of variance.
Ucn1 is a target for miR-326
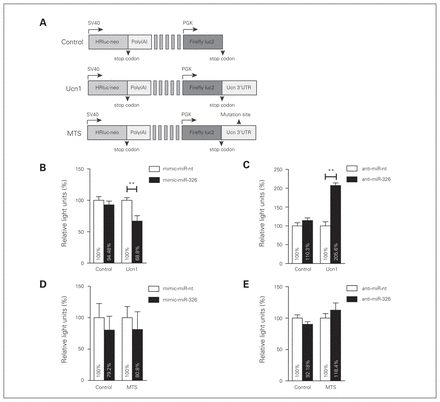
Since ISH experiments in rat brain sections suggested an adjacent colocalization of miR-326 with the Ucn1 neuropeptide in the EWcp region, we investigated whether miR-326 has the capacity to modulate Ucn1 expression levels. To this end, B35 rat neuroblastoma cell lines were cotransfected with either control luciferase plasmid (control-reporter), a luciferase reporter plasmid containing the Ucn1 3′-UTR (Ucn1-reporter), or a luciferase reporter plasmid containing the Ucn1 3′-UTR, in which the putative miR-326 binding site was deleted (mutated targeting site [MTS]–reporter; Fig. 4A). In addition, miR-326 mimics (mimic-miR-326) or inhibitory anti-miR-326 were cotransfected for miR-326 gain or loss of function.
MicroRNA (miR)-326 targets the 3′-untranslated region (UTR) of urocortin 1 (Ucn1) mRNA. (A) PmirGLO plasmid carrying renilla and firefly luciferase reporter genes (Control). The intact Ucn1 3′-UTR (Ucn1) or the Ucn1 3′-UTR lacking the miRNA-binding seed sequence (MTS) were subcloned downstream from a firefly luciferase reporter construct driven by the human phosphoglyserate kinase (PGK) promoter. Renilla expression is driven by an independent Simian vacuolating virus 40 (SV40) early promoter, and is followed by a late SV40 poly(A) region. (B) Luciferase activity for control and Ucn1 plasmids cotransfected with mimic-miR-nt (white bars), or mimic-miR-326 (black bars). Luciferase activity was decreased upon cotransfection of Ucn1 and mimic-miR-326. (C) Luciferase activity for control and Ucn1 plasmids cotransfected with anti-miR-nt (white bars) or anti-miR-326 (black bars). Luciferase activity was increased upon cotransfection of Ucn1 and anti-miR-326. (D) Luciferase activity for control and MTS plasmids cotransfected with mimic-miR-nt or mimic-miR-326 was similar among all conditions. (E) Luciferase activity for control and MTS plasmid cotransfected with anti-miR-nt or anti-miR-326 was similar for all conditions. Firefly luciferase activities were normalized against renilla luciferase activities to calculate relative light units. Error bars represent the standard errors of the mean 8 independent experiments. Relative light units of the control group were considered 100%. **p < 0.01, 1-way analysis of variance.
We first examined whether B35 cells express miR-326 endogenously. Using quantitative PCR we found that the average Ct for this miR was 26 ± 3, raising the possibility that we could use anti-miR-326 to repress its function in these cells. While relative luciferase activity did not change when nontargeting mimic-miR-nt was cotransfected with either the control- or the Ucn1-reporters, overabundance of miR-326 in the presence of the Ucn1-reporter reduced luciferase activity by approximately 30% compared with the control-reporter (Fig. 4B). Conversely, cotransfection of a miR-326 inhibitor (anti-miR-326) with the Ucn1 reporter resulted in increased luciferase activity (Fig. 4C), while luciferase activity did not change when anti-miR-326 was cotransfected with the control-reporter. Results of a deletion experiment confirmed that the 7-mer putative miR-326- targeting site on the 3′-UTR of rat Ucn1 was specifically targeted by this miR-326. Luciferase levels in cells expressing the mutated MTS-reporter did not significantly vary from those of the control-reporter upon either overexpression or inhibition of miR-326 (Fig. 4D and E).
Regulation of neuronal levels of Ucn1 mRNA and protein levels by miR-326
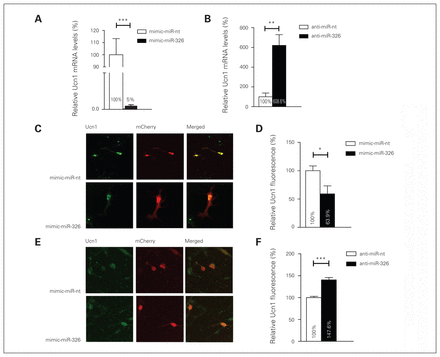
The demonstration that the miR-326 has the capacity to bind to Ucn1 3′-UTR, as revealed by the luciferase gene activity assay, supports the hypothesis that this miRNA plays a role in the regulation of Ucn1 mRNA and protein levels. To explore whether mature miR-326 regulates Ucn1 mRNA levels in rat midbrain neurons, we monitored Ucn1 mRNA levels after transfecting cultured primary midbrain neurons with miR-326 mimics or control miR-nt. Transfection with the miR-326 mimic resulted in an approximately 122-fold increase in mature miR-326 levels compared with the endogenous miR-326 levels in mimic-miR-nt transfected neurons 2 days after transfection (relative miR-326 levels in miR-326 mimics transfected neurons 48.7 ± 0.139; relative miR-326 levels in miR-nt transfected neurons 0.4 ± 0.05, independent t test, 2-tailed p = 0.006, n = 6). The Ucn1 mRNA levels decreased approximately 90% in the mimic-miR-326 transfected neurons compared with neurons transfected with mimic-miR-nt 3 days following transfection (Fig. 5A). Conversely, lipofection of anti-miR-326 into cultured midbrain neurons resulted in a 5-fold increase in Ucn1 mRNA levels, when compared with the anti-miR-nt control, as early as 48 h after transfection (Fig. 5B). Next, we assessed whether miR-326-mediated modulation of Ucn1 mRNA also correlated with an alteration in neuronal Ucn1 protein levels. We found that miR-326 was overexpressed by transfecting an mCherry-miR-326 plasmid into primary midbrain neurons, and Ucn1 protein levels in mCherry-positive neurons were quantified by quantitating the fluorescence intensity upon immunofluorescence staining of Ucn1. Overexpression of miR-326 led to an approximately 45% decrease (p < 0.001) in Ucn1 protein levels in midbrain neurons (Fig. 5C and D). Conversely, inhibition of endogenous miR-326 by cotransfection of anti-miR-326 and an mCherry plasmid led to an approximately 48% increased (p < 0.001) expression of Ucn1 protein in mCherry-positive midbrain neurons (Fig. 5E and F). The outcome this investigation strongly suggests that miR-326 has the capacity to modulate Ucn1 mRNA and protein levels in cultured midbrain neurons.
MicroRNA (miR)-326 regulates urocortin 1 (Ucn1) messenger RNA and protein expression in neurons. (A) In rat primary midbrain neurons, Ucn1 mRNA levels are decreased upon mimic-miR-326 transfection (black bar) compared with mimic-miR-nt transfection (white bar), as determined by quantitative real-time polymerase chain reaction (qRT-PCR)–based quantification. Error bars represent the standard errors of the mean (SEM) for 6 independent experiments. (B) Ucn1 mRNA levels are increased in primary midbrain neurons transfected with anti-miR-326 (black bar) compared with anti-miR-nt transfected neurons (white bar). The relative expression levels were normalized to the expression of the housekeeping genes small nuclear RNA U6 and β-actin. Error bars represent the SEM for 6 independent experiments. (C) Left panels show representative pictures of primary midbrain neurons cotransfected with the mCherry vector together with either miR-nt or miR-326 mimics. (D) Fluorescence-based quantification of Ucn1 protein levels in mCherry-miR-nt (white bar) or mCherry-miR-326 (black bar) transfected midbrain neurons revealed a decrease in Ucn1 protein levels upon miR-326 overexpression. (E) Representative pictures of midbrain neurons cotransfected with the mCherry vector together with either anti-miR-nt or anti-miR-326. (F) Fluorescence-based quantification of Ucn1 protein levels upon transfection with anti-miR-nt or anti-miR-326 demonstrating increased Ucn1 protein levels upon anti-miR-326 transfection. Analysis of immunofluorescence levels was performed by using ImageJ. Error bars represent the SEM for 4 independent experiments, each assessing fluorescence levels of 50–60 neurons. Control relative expression levels were set to 100%. *p < 0.05, **p < 0.01, ***p < 0.001, 1-way analysis of variance.
Discussion
While gene expression regulation by miRNA represents a widespread mechanism of posttranscriptional regulation, to our knowledge, the present study identified for the first time the regulation of a neuropeptide expression by this class of small noncoding RNAs. MicroRNA-326 was first identified among a set of miRNAs with high expression in neurons.37 Intracellular colocalization of miR-326 with its cognate target Ucn1 in the EWcp, along with the conservation of the cis-acting target sequence of for this miRNA on the Ucn1 mRNA 3′-UTR suggests their involvement in fundamental mechanisms regulating the expression of this neuropeptide in response to CVMS. Stress-associated downregulation of miR-326 in the human as well as the rodent midbrain in depression may diminish the rate of Ucn1 mRNA degradation, resulting in increased Ucn1 levels. It is tempting to speculate that high miR-326 expression in neurons is another mechanism by which the stress adaptation response is modulated in the brain. In support of the presented data, it was previously hypothesized that substantial alterations in the posttranscriptional regulatory environment, characterized by changes in miRNA expression patterns in the brain, could have critical implications for the development and pathophysiology of depression-like behaviour.38,39
Previously, we showed that the stress-mediated neuropeptides CART and Ucn1 act upon central stress regulatory pathways and have been shown previously to act together in response to acute stress. Given this notion, we examined the role of miR-326 on modulating both CART and Ucn1, since both of their mRNAs carried a binding site for this miRNA in their 3′-UTRs. The outcome of our studies on the regulation of CART expression regulation by miR-326 is correlative and requires additional attention in future. However, computational analysis calculated a higher p value for the binding of CART to miR-326 than for the binding of Ucn1. Moreover, the miR-326 binding site was not conserved in mice, arguing for a lower probability that CART is regulated by this miRNA. This conclusion, along with Ucn1 being a more physiologically relevant neuropeptide during the induction of the HPA axis, led us to examine Ucn1 as a target of miR-326.
Our study provides direct evidence that miR-326 and Ucn1 are expressed in the EWcp neurons. We also found that relative levels of miR-326 were decreased in the EWcp, whereas levels of Ucn1 mRNA were elevated in rats exhibiting behavioural despair, as judged using the FST. We observed a similar pattern of miR-326 and Ucn1 mRNA expression in depressed suicide completers as that observed in rats. These results reveal comparable alterations in miR-326 and Ucn1 levels in depressed suicide completers and in an animal model for behavioural despair, which is one of the main risk factors for suicidal behaviour.16 To provide experimental evidence for the regulation of Ucn1 by miR-326, we have shown that in vitro overexpression of miR-326 reduces luciferase activity in the presence of the 3′-UTR of Ucn1, while inhibition with anti-miR-326 increases luciferase activity in the presence of the 3′-UTR of Ucn1 compared with the control condition. Although the luciferase assay examined the binding of miR-326 to the rat Ucn1 3′-UTR, the outcome of this experiment suggests that the Ucn1 human homologue is also targeted by this miR. Although noncanonical modes of silencing have been shown for miRNAs in general, many studies have demonstrated that the miR 5′ region binding of the 3′-UTR is sufficient to cause repression, whereas the 3′ region of the miR is less critical. The high conservation among the 5′ regions of human, mouse and rat miR-326 therefore supports the idea that the outcome of luciferase gene activity data would be similar for humans.
The neuronal stress response may be modulated by the action of miRNAs, as previous reports indicated altered miRNA levels upon induction of stress in both humans and rodents.38,40–42 For example, early life stress results in increased expression of a variety of miRNAs in the prefrontal cortex.43 Moreover, miRNA-183 and miR-134 levels are upregulated in the amygdala under acute stress.14 In addition, stress induces a prominent upregulation of miR-34c in the central amygdala in adult male mice, mediating anxiolytic behaviour after challenge by targeting the stress-related corticotropin-releasing hormone receptor 1 (CRFR1) mRNA.44
It is interesting to note that besides the pivotal role miRNAs could play in fine-tuning gene expression in the brain, miRNAs circulating in bodily fluids have recently been applied in the detection of various types of pathologies.45,46 While serum miRNAs cannot cross the blood–brain barrier, previous studies suggested that naturally occurring exosomes are capable of crossing it.47,48 Although relatively little is known about the function and origin of miRNAs in bodily fluids,49 it has been hypothesized that miRNAs are excreted in response to damage or stress,50 and expression of miR-144 and miR-16 has been found to be upregulated in the blood of university students in naturalistic stress situations, such as academic examinations.15 Notably, accumulating experimental and clinical evidence indicates that a number of diseases are associated with blood–brain barrier dysfunctions resulting in elevated barrier permeability, enabling leakage of larger molecules, such as miRNAs, into and out of the brain.51 In line with our finding, the downregulation of miR-326 in CVMS rats points to a possible biosignature application for miR-326. Such a biomarker is worthy of future evaluation in depressed patients. For example, measuring peripheral miR-326 levels in blood or plasma may reveal miR-326 as a reliable biomarker for stress-associated behaviour and suicidal vulnerability. Such a biomarker is important, as the current assessment of suicide risk yields a high sensitivity but a low specificity.52,53 It is expected that using specific neurobiological markers, such as serum miRNA-326, in addition to the clinical assessment may improve the future evaluation of suicide risk, a possibility that needs to be tested in individuals with increased suicidal risk.
Limitations
Upon interpretation of the data presented in this study, a few limitations need to be considered. All controls died of cardiovascular complications, and this could be a potential confounding factor. The significant difference between the age of depressed suicide completers and controls could also be considered as a possible confound. However, it must be noted that in a previous study in which we used the same cohort of postmortem brain samples we found no correlation between Ucn1 and CART mRNA expression. This finding suggests that age may not be a mediator of Ucn1 and CART expression in the EWcp.30 Another factor that needs to be considered is the relatively low number of sample donors included in the study; however, collecting postmortem brain samples of drug-free depressed suicide completers with relatively short postmortem delay and good RNA quality (i.e., the most important factors that could affect mRNA expression levels in postmortem tissue) is challenging.
We also examined the levels of miR-325 in CVMS rats and in suicide completers. Although CART mRNA contains a nonconserved binding site for this miR, the expression levels among humans and CVMS rats were not consistent, as we could not detect significantly altered miR-325 levels in suicide completers compared with deceased controls. Because of these observations we opted to focus on the regulation of Ucn1 by miR-326.
In the present study we did not show that in vivo alteration of miR-326 levels would result in a improvement in behavioural despair in the FST. In this regard, virus-mediated miR-326 sponge delivery into rat midbrain regions failed to demonstrate a definitive repression of miR-326 in midbrain neurons after 14 days of viral infection. A possible explanation for our observation can be that neurons infected by the miR-326 sponge virus had an necrotic phenotype, whereas cultured neurons transfected with miR-326 mimics or anti-miR-326 remained healthy. Accumulating evidence suggests that alteration of miR-326 levels results in apoptosis in glioma cells54 and is dysregulated in different cancers.55,56
Conclusion
The clinical importance of our study goes well beyond the miR-326-mediated regulation of Ucn1 expression in the midbrain; our findings point toward a novel mechanism for the regulation of neuropeptide expression by miRNAs. To our knowledge, the present study is the first to demonstrate such a regulatory action for any miRNA and therefore adds to the plethora of functions already linked to miRNAs. As neuropeptides play a very important role in stress-associated neuropathologies, identification of neuropeptides as direct targets of miRNA-mediated expression regulation are likely to reveal novel mechanisms for the stress adaptation response. This could advance our understanding of the underlying mechanisms of suicidal behaviour and of suicide risk. Given miRNAs are excellent drug target candidates, our findings might ultimately lead to the discovery of novel treatment options for a substantial number of individuals manifesting suicide vulnerability.
Acknowledgements
The authors thank the individuals who provided technical assistance. This work was supported by a Donders Center for Neuroscience fellowship award of the Radboud University Nijmegen Medical Centre and a grant from FP7-Marie Curie International Reintegration to A. Aschrafi.
Footnotes
Competing interests: J. Glennon declares consulting fees from Boehringer Ingelheim outside the submitted work. No other competing interests declared.
Contributors: A. Aschrafi, M. Palkovits, B. Gaszner and T. Kozicz designed the study. All authors acquired the data, which A. Aschrafi, J. Verheijen, P. Gordebeke, G. Martens, B. Gaszner and T. Kozicz analyzed. A. Aschrafi, J. Glennon, B. Kaplan and T. Kozicz wrote the article, which all authors reviewed and approved for publication.
- Received May 3, 2015.
- Revision received September 21, 2015.
- Accepted December 14, 2015.